Traumatic Ventral Cord Herniation Presenting with Sexual Dysfunction: A Case Report and Review of Literature
Omar Al-Awar2,4, Georgio Haddad2,3*, Tarek Bou Dargham3, Mohamad Bahij Moumneh3, Elias Horanieh3, Christine Atallah3, Mira Bou Ayache5, Marwan M Haddad6 and Churl-Su Kwon1
Omar Al-Awar2,4, Georgio Haddad2,3*, Tarek Bou Dargham3, Mohamad Bahij Moumneh3, Elias Horanieh3, Christine Atallah3, Mira Bou Ayache5,Marwan M Haddad6 and Churl-Su Kwon1
1Departments of Neurological Sciences, Columbia University Medical Center, NewYork
2Department of Neurosurgery, University of Balamand University Medical Center, Mount Lebanon Hospital, Hazmiyeh, Lebanon
3Department of Medicine and Medical Sciences, University of Balamand, El-Koura, Lebanon
4Department of Neurosurgery, Ain Wazien Medical Village University Hospital, Ain w Zain, Lebanon
5Department of Medicine, Lebanese American University, Beirut, Lebanon
6Department of Radiology, University of Balamand University Medical Center,Mount Lebanon Hospital, Hazmiyeh, Lebanon
*Corresponding Author:
- Georgio Haddad, Department of Neurosurgery, University of Balamand University Medical Center, Mount Lebanon Hospital, Hazmiyeh, Lebanon E-mail:m_haddad@hotmail.com
Received date:June 14, 2022, Manuscript No. IPRDDT-21-13647; Editor Assigned date: June 16, 2022, PreQC No. IPRDDT-21-13647 (PQ);Reviewed date: June 29,2022 QC No. IPRDDT-21-13647; Revised date:July 07, 2022, Manuscript No. IPRDDT-21-13647 (R); Published date: July 14, 2022, DOI: 10.36648/2380-7245-8.7.57
Citation: Awar OA, Haddad G, Dargham TB, Bahij Moumneh M and Horanieh E et al. (2022)Traumatic Ventral Cord Herniation Presenting with Sexual Dysfunction: A Case Report and Review of Literature. J Rare Disord Diagn Ther Vol.8 No.6: 57.
Abstract
Spinal cord herniation is a characteristic bulging from a defective arachnoid membrane and dura mater that is generally regarded as a rare neurosurgical case. Acknowledging that there are fewer than a hundred cases worldwide, this case report portrays a thirty-year-old male with diminished tactile, pinprick, and temperature sensation in the left lower and upper limbs consistent with Brown-Sequard syndrome presenting with a history of erectile dysfunction. This case report aims at raising awareness towards spinal cord herniations to better manage and treat this rare condition. As a treatment option, the patient underwent a surgical decompression using laminectomy to repair the dural defect with a synthetic dura mater below the level of T8. The implication of this case is to show that although ventral cord herniation associated with erectile dysfunction is a rare neurological case, it should be kept in the mind of physicians when faced with atypical cases of myelopathy that are not consistent with frequent etiologies. We show that such cases can be treated with surgical intervention, as it is imperative to patient treatment and recovery.
Keywords: Ventral cord; Herniation; Operative technique; Spinal cord surgery; Trauma
Introduction
Wortzman first identified Spinal Cord Herniation (SCH) in a case report in 19741. SCH is the outward displacement of the spinal cord through a dural sac defect of the arachnoid membrane and the dura mater with the thoracic cord being the most affected region [1-4]. SCH remains a rare pathological entity encountered in the field of neurosurgery; however, it is a treatable spinal cord disease of unknown pathophysiology that is often missed as a diagnosis for paraplegia or neurological impairment [5,6]. The term Thoracic Anterior Cord Adhesion Syndrome (TASCS) is used in mild cases [7]. Whereas in advanced or severe cases, the spinal cord is protruded through a ventral dural defect where the bulge or the protrusion can sometimes reach centimeters through it.
Fewer than 100 cases have been reported in the literature, but the exact pathogenesis of SCH remains unknown. The literature contains several proposed theories in an attempt to explain the pathological mechanism of SCH. A study by Francis et al. claimed that it may be due to a congenital dural deficiency or related to a history of traumas [8]. Other authors have proposed an etiological basis related to pressure erosion of the dura, dural fistula, trans-dural disk herniation, spinal tumor or spinal arachnoid cysts dislocating the spinal cord [9], and duplication of the ventral dura [6,10-12]; as well as spontaneous or idiopathic, and iatrogenic cases [2,13-16]. Nonetheless, traumatic spinal-cord herniation is poorly documented in the literature [8,17].
Typical MRI imaging investigations of the previously proposed etiologies demonstrate focal anterior ventral cord herniation between T2-T8 without intervening Cerebrospinal Fluid (CSF) and a dilated posterior CSF compartment similar to an arachnoid cyst [18-20]. In fact, an arachnoid cyst is ruled out as a probable cause in the presence of normal unobstructed CSF flow. Usually, arachnoid cysts tend to displace the nerve roots peripherally where it is best seen on a high-resolution T2-weighted imaging, making it a helpful tool in distinguishing a cyst from herniation [21]. When MRI results are inconclusive, a CT myelography is required as it shows unrestrained flow of contrast through the dural defect at the level of the herniation or a widened dorsal subarachnoid space [22]. Both imaging modalities led to the development of an idiopathic SCH classification system that contributed to decision-making in the management of SCH and the establishment of a prognosis. This classification includes several types: Type K (kink) where the imaging shows a kink to the ventral region, Type D (discontinuous) where the spinal cord is no longer evident at the herniated site, and Type P (protrusion), in which the subarachnoid space of the ventral spinal cord vanishes without a kink [4,23].
The neurological impairment of SCH most commonly appears between the ages of 22-71, with females being more commonly affected [1,24]. Patients with SCH present with progressive spastic paraparesis or Brown-Séquard syndrome, where the latter can also cause numbness, weakness in the legs, walking difficulty, as well as problems with bowel and bladder function [4,25-27].
There is no consensus on the surgical technique used to manage this condition as several have been reported in literature with the common aim of reducing the herniation and preventing recurrence [18,20]. As demonstrated by several studies, primary closure using sutures showed a high rate of post-surgical complications and thus yielded poor outcomes as opposed to the posterior approach whose main mechanism is the destruction of the dural defect and the use of a synthetic dural graft [28-30].
In this study, we report a case of a 30-year-old male who presented to Ain Wazein medical village, Lebanon, with sexual dysfunction and neurological impairment post trauma. The aim of this study is to raise the attention to such a rare condition in order to establish a better understanding of its mechanism and management [31].
Case Report: Presentation, Examination, & Follow-up
A 30-year-old young male presented with a history of progressive sexual dysfunction, burning sensation, and motor weakness in the left lower limb. Ten and a half years prior to presentation, the patient was admitted to a local hospital for trauma and underwent a Computer Tomography (CT) of the thoraco-lumbar spine. The result was negative, and he was discharged home with analgesics for back pain. However, his complaints persisted and three months prior to presentation, he experienced weakness and a loss of perception of temperature sensation in his left leg, especially while walking. Additionally, bladder and bowel functions were normal.
A neurological examination revealed Brown-Séquard syndrome below the level of T8. He had diminished tactile and pinprick sensation and reduced temperature sensation in the left lower extremity. His sense of position and vibration were bilaterally preserved, and reflexes were all brisk.
Following surgery, the patient regained sensation in his upper limbs and chest, in addition to an improvement in his erectile sexual dysfunction (Figure 1).
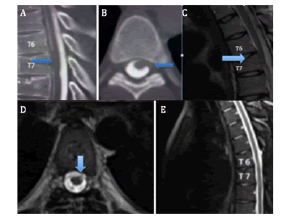
Figure 1: A,B) Preoperative CT myelography showing no contrast ventral to the spinal cord at T6–7. A) Sagittal image demonstrating contrast enhancement dorsal to the herniated spinal cord. B) An axial image demonstrating the ventral herniation at the level of the disc space. C) A sagittal T2-weighted image demonstrates a ventral spinal cord herniation at the level of T6-7. D,E) Postoperative MR imaging study of the thoracic spine demonstrating realignment of the spinal cord within the canal and restoration of CSF ventral to the spinal cord.
Surgical Procedure
General anesthesia was administered to the patient in prone positioning with perioperative steroids and antibiotics. In addition, normotensive intraoperative blood pressure goals were reviewed with the anesthesia team. Prior to surgery, the patient was marked by injecting methyl at the level of the cord herniation with CT guidance. The anatomic levels were double checked prior to the skin incision with fluoroscopy and the posterior thoracic region was prepared with antiseptic solutions in the proper fashion.
Skin and dorsal fascia were opened in the midline and the paraspinal muscles were reflected from the spinous process and lamina. To better expose the spinal cord and identify the location of the displacement, a thoracic laminectomy and intraoperative ultrasound were performed, respectively. The dura was opened in the midline and secured with retractive sutures. Then, a careful microdissection of the arachnoid and generous freeing of the arachnoid adhesion from the upper and lower poles of the herniated spinal cord were conducted until a normal anatomical position was achieved.
Once a sufficient path around the cord at the level of the dural defect was achieved, a square piece of artificial dura was advanced circumferentially around the inner aspect of the dura, anterior to the cord (Figure 2). Double-checking at this stage is crucial to confirm an adequate reduction of the hernia and no undue pressure or tension on the spinal cord at the level of the existing nerve roots. After achieving an adequate cord reduction, the dura and wound were closed in a routine fashion, including the use of a dural sealant.
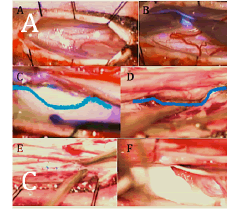
Figure 2: A) Dura opened post laminectomy. B) Careful retraction of the spinal cord and cutting the dentate ligament bilaterally to manipulate the spinal cord. C) Margin of the dura (blue line) of the spinal cord above the line and the dural edge below the line. D) Post release of the spinal cord ventrally from the dural defect. E) Suturing the floor of the dural defect with 6.0 nylon. F) Placement of DuraGen above the suture line.
Literature Review
PubMed and Medline libraries were searched using the term “ventral cord herniation”. Case reports and literature reviews published between the years 2000-2021 were selected. The level of the herniation, cause, symptoms, surgical technique, and follow-up were extracted from every case (Table 1).
| Year | Author | Age/sex | Localization | 1st appearance of symptoms | Symptoms | Cause | Management | Surgical technique | Post-operation outcome |
|---|---|---|---|---|---|---|---|---|---|
| 2000 | Ewald et al (31) | 51/F | T6 | 2 years PTP | Brown-Séquard syndrome | Idiopathic | Surgery | T6 laminectomy | Improvement of neurological symptoms |
| 2001 | Eguchi et al(32) | 49/F | T4-T5 | 10 years PTP | Left lower limb weakness | Idiopathic | Surgery | T4-T5 laminectomy | Formation of a compressive arachnoid cyst and worsening of the symptoms |
| 2001 | Miyaguchi et al(9) | 54/F | T3-T4 | 2 years PTP | Brown-Séquard syndrome | Idiopathic | Surgery | T3-T4 laminectomy | Gradual improvement in strength |
| 2001 | Pereira et al(33) | 55/M | T2-T3 | 4 years PTP | Left lower limb weakness | Idiopathic | Surgery | T2-T3 laminectomy | Uneventful |
| 2001 | Aizawa et al(34) | 44/M | T8-T9 | 5 years PTP | Left lower limb numbness | Idiopathic | Surgery | T8-T9 laminectomy | Muscle power recovered 1-year /post-op |
| 2001 | Aizawa et al(34) | 60/F | T4-T5 | 3 years PTP | Brown-Séquard syndrome | Idiopathic | Surgery | T3-T6 hemilaminectomy | Motor power improved/sensory showed no improvement |
| 2001 | Aizawa et al(34) | 59/F | T4-T5 | 20 years PTP | Left lower limb weakness | Idiopathic | Surgery | T3-T6 laminectomy | Motor power improved/sensory showed no improvement |
| 2001 | Berbel et al(35) | 56/M | N/A | N/A | Brown-Séquard syndrome | Idiopathic | Surgery | Laminectomy | Mild improvement |
| 2001 | Adams et al(36) | 56/M | T7 | 6 months PTP | Brown-Séquard syndrome | Trauma | None | N/A | Progression of the herniation and increase of the weakness |
| 2003 | Nakagawa et al(37) | 77/F | T6-T7 | 9 years PTP | Urinary incontinence and weakness in LL | Trauma | Surgery | T6-T8 laminectomy | Aggravation of the symptoms 2 months post-op |
| 2003 | Nakagawa et al(37) | 77/F | T6-T7 | 9 years PTP | Recurrence of symptoms | Trauma | Surgery | T6-t8 laminectomy | Symptoms improved 1 year postop |
| 2003 | Sagiuchi et al(38) | 48/M | T7-T8 | 20 years PTP | Gait difficulties | Idiopathic | Surgery | T7-T8 Laminectomy | Improvement in motor and sensory functions |
| 2004 | Najjar et al(39) | 32/M | T8-T9 | 11 years PTP | Brown-Séquard syndrome | Idiopathic | Surgery | T9-T9 laminectomy | Regained normal gait |
| 2004 | Rivas et al(40) | 49/M | T6-T7 | N/A | Brown-Séquard syndrome | Idiopathic | Surgery | T6-T7 laminectomy | Motor power improved/sensory showed no improvement |
| 2004 | Spissu et al(24) | 56/F | T7 | 14 months PTP | Brown-Séquard syndrome | Trauma | Surgery | Lysing of adhesion and relocating the spinal cord to its anatomical position | Uneventful (motor power improved/sensory showed no improvement) |
| 2004 | Maruichi et al(41) | 53/M | T4-T5 | N/A | Numbness and pain of the RLL | Idiopathic | Surgery | T3-T5 laminectomy | N/A |
| 2004 | Aquilina et al(42) | 37/F | T4 | N/A | N/A | Idiopathic | Surgery | Surgical exploration and reduction | Significant improvement |
| 2005 | Ferre et al(43) | 70/M | T10-T11 | 28 months PTP | Slowly progressive gait deterioration | Idiopathic | Surgery | T10-T11 laminectomy | Rapid improvement for both sensory and motor |
| 2005 | Ferre et al(43) | 75/F | T5-T6 | 1-year PTP | Brown-Séquard syndrome | Trauma | Surgery | T5-T6 laminectomy | No significant change in the neurological status |
| 2005 | Ferre et al(43) | 48/F | T5-T6 | 4 years PTP | Paresthesia LLL | Idiopathic | Medical management | N/A | Stable with symptomatic improvement |
| 2006 | Darbar et al(44) | 41/M | T5 | 3 years PTP | Brown-Séquard syndrome | Trauma | Surgery | T4–T6 laminectomy | Improvement of gait and bladder control |
| 2006 | Darbar et al(44) | 63/F | T6-T8 | 15 years PTP | Brown-Séquard syndrome | Trauma | Surgery | Repair of the Dural defect and untethering of the cord | Resolution of urinary symptoms, and partial amelioration in the RLL |
| 2006 | Darbar et al(44) | 34/F | T7-T8 | 3 years PTP | Brown-Séquard syndrome | Idiopathic | Surgery | T7-T8 Laminectomy | Paraparesis improved after surgery. |
| 2006 | Morley et al(45) | 28/F | T5-T6 | 2 years PTP | RLL weakness and difficulty walking. | Idiopathic | Surgery | Cord reduced and the defect repaired with a synthetic graft. | Motor weakness improves, sensory deficit persisted |
| 2006 | Inoue et al(46) | 71/F | T2-T3 | N/A | Brown-Séquard syndrome | Idiopathic | Surgery | T1-T3 laminectomy | Slight neurological improvement |
| 2006 | Saito et al(47) | 57/M | T2-T3 | Brown-Séquard syndrome | Idiopathic | Surgery | Repair of the Dural defect | Improvement of the patient symptoms | |
| 2006 | Bandai et al(48) | 63/F | T2-T3 | 5 years PTP | Slowly progressive gait disturbances | Idiopathic | Surgery | T2-T3 laminoplasty | No improvement |
| 2008 | Alkan et al(49) | 36/M | T5-T6 | 9 years PTP | Bilateral paraparesis, lumbar pain, urinary incontinence, and erectile dysfunction | Idiopathic | Conserva-tive management | N/A | Na -stable neurologically |
| 2008 | Ghostine et al(50) | 47/F | T6-7 | 3 years PTP | Low back pain, progressive myelopathy, right proximal LLL deficit, sensory deficit, and pathologic reflexes. | Idiopathic | Surgery | Laminoplasty and intradural exploration | Improvement |
| 2008 | Selviaridis et al(15) | 51/M | T2-T3 | 2 years PTP | Progressive weakness and sensory disturbances in the RLL and left paresthesia | Idiopathic | Surgery | T2–T3 laminectomy and reduction of the herniated cord after adhesiolysis from the arachnoid cyst | Follow up at 2 years post: cured, returned to normal state. |
| Recurrence of herniation 10 years post-op | |||||||||
| 2008 | Senturk et al(51) | 38/F | T4 | 6 months PTP | Chest pain radiating through to the back at the T4 dermatome bilaterally. | Idiopathic | Non-operative | N/A | N/A |
| 2009 | Sasani et al(52) | 45/F | T-8 | 2 years PTP | Brown-Séquard syndrome below the level of T8 | Idiopathic | Surgery | Laminectomy at T8 | Improvement |
| 2011 | Nakamura et al(53) | Duration of disease: | Brown-Séquard,Brown-Séquard,Brown-Séquard,paraplegia,Brown-Séquard,Brown-Séquard,paraplegia, paraplegia, Brown-Séquard,Brown-Séquard,Brown-Séquard,Brown-Séquard,Brown-Séquard,paraplegia | Idiopathic | Surgery | N/A | Recovery (%): 57,43,43,0,50,50,22,50,40,40,17,67,67,67,60,25 JOA score increased in 15 post-op out of 16 patients | ||
| 5 years, | |||||||||
| 43 N/A, | T4, | 3 years, | |||||||
| 39 N/A, | T3, | 4 years, | |||||||
| 54 N/A, | T4, | 10 years, | |||||||
| 71 N/A, | T4, | 5 years, | |||||||
| 49 N/A, | T4, | 5 years, | |||||||
| 47 N/A, | T5, | 16 years, | |||||||
| 78 N/A, | T4, | 2 years, | |||||||
| 56 N/A, | T6, | 3 years, | |||||||
| 47 N/A, | T3, | 1 year, | |||||||
| 46 N/A, | T4, | 8 years, | |||||||
| 68 N/A, | T7, | 3 years, | |||||||
| 67 N/A, | T4, | 1 year, | |||||||
| 42 N/A, | T3, | 0.5 years, | |||||||
| 53 N/A, | T5, | 3 years, | |||||||
| 60 N/A, | T5, | 3 years | |||||||
| 68 N/A | T3 | ||||||||
| 2011 | Liu et al(54) | 56/M | T-11, | 18 months PTP | Progressive difficulty in walking and numbness in both lower limbs | Idiopathic | Patient refused operation | N/A | N/A |
| T-12 | |||||||||
| 2012 | Akutsu et al(55) | 50/M 66/F 83/F | T6-T7, T4-T5, T5-T6 | 60 months 120 months 120 months | Brown-séquard, brown-séquard, paraparesis | Idiopathic | Surgery | Laminectomy at the level of herniation | Recovery rate: 50%, 38%, 12.5% JOA post-op score increased in all 3 patients |
| 2013 | Summers et al(6) | 66/M 51/F 81/M | T5 T7 T3-T5 | 18-year history 3-year history of back pain 10 days | Upper thoracic spine pain | Idiopathic | Conserva-tive manage-ment | N/A | N/A - 1st case: stable neurologically at 4 months post presentation 2nd case: stable neurologically at 4 years post presentation 3rd case: stable neurologically at 3 months after presentation |
| Thoracic back pain | |||||||||
| Progressive bilateral LL weakness and generalized numbness in his thorax and LLs. | |||||||||
| 2013 | Moriyama et al(56) | 51/M | C-7 | 10 years PTP | Progressive paraparesis and urinary disturbance | Post-operative (spinal tumor) | Surgery | Adhesiolysis at the spinal cord and the Dural defect and then duroplasty of the Dural defect | Improvement |
| 2013 | Krishnan et al(57) | 50/F | T6-T7 | 3 years PTP | Slowly progressive gait difficulties | N/A | Surgery | Laminectomy, reduction of the cord with micromanipulation and then duroplasty | Improvement |
| 2014 | Berg-Johnsen et al(4) | 44.6/F | T4/5 | 3 years | Paraparesis sensory level | Idiopathic | Surgery | Laminectomy or laminoplasty | Improved |
| 63.9/F | T5/6 | 5 years | Brown-Séquard | No change | |||||
| 75.5/M | T4/5 | 4 years | Paraparesis sensory level | Improved (transient) | |||||
| 58.3/F | T4/5 | 3 years | Brown-Séquard bladder dysfunction | Improved (slightly) | |||||
| 57.1/F | T4 | 6 years | Brown-Séquard bladder dysfunction | Improved | |||||
| 42.0/F | T6/7 | 2 years | Brown-Séquard bladder dysfunction | No change | |||||
| 60.0/F | T7/8 | 10 years | Improved | ||||||
| 2014 | Yamamot-o et al(58) | 60/F | T5-T6 | 15 years PTP | Brown-séquard | Idiopathic | Surgery | Laminectomy and right-sided partial pediculectomy of the t5 and t6 vertebrae was performed | At 2 years follow up, the patient had no recurrence of symptoms, no instability, and no back pain. |
| 2014 | De Souza et al(59) | 66/F | T-4 | 7 years PTP | Back pain | Idiopathic | Surgery | Laminectomy with reduction of the hernia and ventral Dural repair | Improved |
| 2017 | Lui et al(60) | 62/M | T-2/T-3 | 2 years PTP | LLL deficit, hypersensitivity, hypertonia, and gait abnormality | Idiopathic | Surgery | T-2/T-3 laminectomy with Dural patch and dentate ligament hitch stitches | Hyperesthesia remained |
| 2017 | Lui et al(60) | 42/F | T-5/T-6 | 10 years PTP | Progressive motor deterioration, pain around the rib cage, spastic gait | Idiopathic | Surgery | T-5/T-6 laminectomy with Dural patch and dentate ligament hitch stitches | Improved |
| 2017 | Ronald et al(61) | 52/M | T-4/T-5 | 10 years PTP | Sensory deficit, motor deficit, paresthesia in the LLL | Traumatic injury | Surgery | T-4/T-5 laminectomy | LLL improved |
| RLL unchanged | |||||||||
| 2017 | Ronald et al(61) | 58/F | T-8 | 1-year PTP | Temperature changes, motor deficit, and hyperpathia in LLL. | Idiopathic | Surgery | T-7/T-8 laminectomy | Unchanged |
| 2017 | Delgado-Lopez et al(62) | 33/F | T-7/T-8 | 20 months PTP | Brown-séquard | Idiopathic | Surgery | T-7/t-8 laminectomy and closure of the Dural defect with titanium micro staples | Recovered |
| 2017 | Shimizu et al(63) | 33/M | T-5/T-6 | 6 months PTP | Progressive gait disturbances | Idiopathic | Surgery | T-5/T-6 laminectomy | Motor deficit improved; sensory deficit persisted |
| 2018 | Ghali et al(64) | 66/F | T-5/T-6 | 3 years PTP | Progressive leg spasticity and urinary incontinence | Idiopathic | Surgery | Laminectomy | Improved |
| 2018 | Bartels et al(65) | 24/M | T-4 | N/A | Left sided Brown-Sequard Syndrome | Develop-mental disorder | Surgery | Spinal cord untethering | The patient recovered without deficit |
| 2019 | Tyagi et al(5) | 72/M | T-4/T-5 | 35 years PTP | Progressive stiffness and weakness of his LLs. JOA 4 of 11. | Idiopathic | Surgery | T3-T5 laminectomy, midline durotomy, reduction of cord dislocation then ventral Dural patch placement. | 1 year follow-up JOA 4 of 11 |
| 2019 | Tyagi et al(5) | 31/F | T-3/T-4 | 4 years PTP | Upper back pain, decreased temperature sensations on the LLL with stiffness and weakness. | Idiopathic | Surgery | T3-T4 laminectomy with partial left facetectomy. Durotomy, then spinal cord was reduced. Duroplasty ventrally | Improved motor power with mild deficit. JOA 10 of 11. |
| 2019 | Herring et al(66) | 72/F | T-8/T-9 | 1-year PTP | Brown-séquard | Idiopathic | Surgery | T-8/T-9 laminectomy | Neurological symptoms improved without complications |
| 2019 | Neale et al(67) | 61/F | T-4 | 4 years PTP | Brown-séquard | Idiopathic | Surgery | T-3/T-4 laminectomy | Worsened |
| 2020 | Lunes et al(28) | 55/F | T-4/T-5 | 3 years PTP | Back pain, LLL weakness, urinary incontinence | Idiopathic | Surgery | Surgical decompression | Partial improvement |
| 2020 | Finneran et al(68) | 49/M | C-5 | 1-year PTP | Neck pain, headache, paresthesia in the ULs | Iatrogenic (C5 corpect-omy) | Surgery | C4-C6 vertebral corpectomy, reduction and anatomic realignment of the spinal cord, then C3-C7 reconstruction and fusion | Motor and sensory power restored in all 4 limbs 3 months after. |
| 2020 | Bakhshes-hian et al(3) | 60s/M | T-4 | N/A | Sensory deficit in RLL, weakness and spasticity in LLL. Gait instability. | Idiopathic | Surgery | A T3-T4 laminectomy, left-sided facetectomy, and left-sided T4 pediculectomy, spinal cord reduction | Neurological symptoms improved, residual LLL weakness |
| 2020 | Randhaw-a et al(11) | 50/M | T-2 | N/A | Progressive myelopathy | Idiopathic | Surgery | Laminectomy | Improved |
| 2020 | Aljuboori et al(69) | 35/F | T-4 | 1-year PTP | LLs weakness and numbness | Idiopathic | Surgery | N/A | Improved |
| 2020 | Regensb-urger et al(70) | 51/M | T-2/T-3 | 2004: right trunk pain 2012: spastic deficit of the RLL | 2012 progressive spastic paresis of RLL associated with Babinski sign | Idiopathic | Surgery | Laminectomy | MEP unchanged. SEP slightly improved |
| 2021 | Diaz et al(71) | 38/M | C-3/C-4 | N/A | Progressive cervical myelopathy | Iatrogenic (schwan-oma resection) | Surgery | C1-C5 laminectomy | Sensory and motor improvement |
| 2021 | Teng et al(72) | 48/F | T-3/T-4 | 6 months PTP | Progressive non-radiating thoracic back pain associated with migraines. RLL weakness. | Idiopathic | Surgery | T-3/T-4 laminectomy | LLL weakness post-op improved later. Mild RLL deficit persisted. |
| 2021 | Wilson et al(73) | 48/F | T-7/T-8 | 3 years PTP | Cerebral palsy, worsening myelopathy, gait ataxia | Traumatic | Surgery | T-7/T-8 laminectomy | Improved |
Results
Throughout the literature review, 51 articles describing spinal cord herniation between the years of 2000 and 2021 were retrieved and the cases are summarized in Table 1. The data shows that the thoracic region of the spinal cord is the most commonly affected area. Exceptions include 3 case reports involving the cervical regions C3, C4, C-5 and C-756, 68, 71. Most of the patients described in the articles presented with symptoms that could be described as Brown-Séquard. However, as shown in the table, the timeframe between the development of the very first symptoms and the time of presentation and surgery varied widely. It can also be noted that the patients’ severity of symptoms also differed and ranged from mild to severe impairment. Sixty-one patients were described with 72% of them diagnosed with idiopathic spinal cord herniation, 20% with spinal cord herniation attributed to a previous spinal cord injury, and lastly, 5% with spinal cord herniation attributed to prior surgery involving the spinal cord. Ninety percent of the patients underwent surgical intervention with laminectomy, while a few cases were followed clinically. Upon follow-up, most of the patients’ symptoms improved post-operatively, whereas a few cases reported worsening of the symptoms or no change at all [4,32,33,36,37,40,43,60,61,65,67].
Discussion
Several conditions might lead to the rare onset of a spinal cord herniation such as dural and arachnoid membrane defects or rupture of the cord adhesions from their surrounding meninges. After Wortzman et al. [1] described the first incarcerated SCH in 1974, several cases have been reported and classified into subclasses of varying etiologies generating this condition. The main etiologies described in the literature are congenital, iatrogenic, idiopathic, and post-traumatic [2,7-9,27,74]. Idiopathic spinal cord herniation is the most common and well-defined entity occurring predominantly in the thoracic cord. This class is frequently associated with calcified disc fragments which, in theory, may have caused dural fragilization and microtears. The thoracic localization of SCH might be attributed to the length and weight carried by the thoracic spine [7,74] because it has a smaller diameter when compared to the cervical or lumbar spine and more room inside the dural sac surrounding it, making its mobilization, and kinking easier. Iatrogenic herniation, where dural and meningeal envelopes are weakened by traumatic surgical manipulation, have been reported secondary to a failure in a C1–C2 fixation (wiring), as well as surgeries performed for cervical stenosis that are complicated post-op by pseudomeningocele [75-77]. Post-traumatic herniation has been noted secondary to a nerve root avulsion, penetrating injury to the dura, and vertebral fractures. The following work will discuss traumatic spinal herniation cases that are usually not as well documented in the literature as others [8,17,78-81].
Most of the case reports reviewed in the literature have demonstrated progressive neurological deficits developing years after the initial injury. This was particularly seen in our case where the trauma occurred 10.5 years prior to presentation, suggesting a progressive mechanism of cord herniation. This mechanism is attributed to the contact between the defective part of the dura and the epidural space which generate adhesions with the cord. This, in turn, causes a shift of the cord placement in the dural sac towards the defect, leaving empty space filled with Cerebrospinal Fluid (CSF) that is sometimes mistaken to be an arachnoid cyst26. Subsequently, cord adhesions with the meninges occur, the denticulate ligament is loosened, and CSF pulsations progressively squeeze a segment of the cord through the defect [7,14,15]. This could possibly explain the progressive aggravation of the symptoms in our patient’s case, where his condition worsened three months before arriving at our institution.
The most common presentation of idiopathic spinal cord herniation is Brown-Sequard syndrome [26]. This may be due to the fact that dural defects in SCH cases are mainly anterior and antero-lateral, and that the part of the cord that is suffering from the compression, the kinking or the vascular compromise is limited to a lateralized hemi section of the cord at that level. Although this is found in idiopathic SCH, it was also found in our patient’s case of post traumatic SCH. Most of these cases in literature were found to have caused diffuse myelopathy or unilateral pyramidal symptoms as the most common presenting symptoms. Myelopathy or myeloradiculopathy can also be seen in iatrogenic herniation [81]. In our case report, the patient was mainly suffering from progressive sexual dysfunction, burning sensation in the left lower limb, as well as motor weakness.
MRI is the gold standard investigation for diagnosing SCH with typical features demonstrated on both axial and sagittal images. Sagittal images demonstrate expansion of the dorsal subarachnoid space, and a ventrally displaced spinal cord with anterior C- or S-shaped angulation. On axial images, there is antero-lateral displacement of the cord with loss of the normal intervening CSF signal [7,19]. These features were classically seen in our case.
Conclusion
Ventral cord herniation is a complex disease, in which the spinal cord herniates through defective meninges. Its pathophysiology is complex and has multiple etiologies. Although rare, it should be taken into consideration when confronted with atypical cases of myelopathy with neurological deficits that does not pertain to the classic pathologies.
References
- Wortzman G, Rewcastle NB, Tasker RR, Richardson JC, Pearson FG (1974) Spontaneous incarcerated herniation of the spinal cord into a vertebral body: A unique cause of paraplegia. J Neurosurg 41: 631–635.
[Crossref], [Google Scholar], [Indexed]
- Aizawa T, Sato T, Tanaka Y, Kotajima S, Sekiya M, et al. (2001) Idiopathic herniation of the thoracic spinal cord: Report of three cases. Spine 26: 488-491.
[Crossref], [Google Scholar], [Indexed]
- Bakhsheshian J, Strickland BA, Liu JC (2020) Ventral thoracic spinal cord herniation: Clinical image and video illustration of microsurgical treatment. World Neurosurg 142: 152-154.
[Crossref], [Google Scholar], [Indexed]
- Berg-Johnsen J, Ilstad E, Kolstad F, Zuchner M, Sundseth J (2014) Idiopathic ventral spinal cord herniation: An increasingly recognized cause of thoracic myelopathy. J Cent Nerv Syst Dis 6: 85.
[Crossref], [Google Scholar], [Indexed]
- Tyagi G, Prabhuraj AR, Bhat DI, Rao MB, Devi BI (2019) Duplication of ventral dura as a cause of ventral herniation of spinal cord-a report of two cases and review of the literature. World Neurosurg 126: 346-353.
[Crossref], [Google Scholar], [Indexed]
- Summers JC, Balasubramani YV, Chan PCH, Rosenfeld J V (2013) Idiopathic spinal cord herniation: Clinical review and report of three cases. Asian J Neurosurg 8: 97.
[Crossref], [Google Scholar], [Indexed]
- Taylor TR, Dineen R, White B, Jaspan T (2012) The thoracic anterior spinal cord adhesion syndrome. Br J Radiol 85: 123-129.
[Crossref], [Google Scholar], [Indexed]
- Francis D, Batchelor P, Gates P (2006) Posttraumatic spinal cord herniation. J Clin Neurosci 13: 582-586.
[Crossref], [Google Scholar], [Indexed]
- Miyaguchi M, Nakamura H, Shakudo M, Inoue Y, Yamano Y (2001) Idiopathic spinal cord herniation associated with intervertebral disc extrusion: A case report and review of the literature. Spine 26: 1090-1094.
[Crossref], [Google Scholar], [Indexed]
- Eli I, Guan J, Karsy M, Mazur MD, Dailey A (2019) A case of ventral spinal cord herniation from a chronic dural-pleural fistula resulting in thoracic myelopathy. Cureus 11: 6123.
[Crossref], [Google Scholar], [Indexed]
- Randhawa PS, Roark C, Case D, Seinfeld J (2020) Idiopathic spinal cord herniation associated with a thoracic disc herniation: Case report, surgical video, and literature review. Clin spine Surg 33: 222-229.
[Crossref], [Google Scholar], [Indexed]
- Chellathurai A, Balasubramaniam S, Gnanasihamani S, Ramasamy S, Durairajan J (2018) Pathophysiology and grading of the ventral displacement of dorsal spinal cord spectrum. Asian Spine J 12: 224-231.
[Crossref], [Google Scholar], [Indexed]
- Castelnovo G, Hladky JP, Renard D (2014) Spontaneous transdural spinal cord herniation. Neurology 82:1290.
[Crossref], [Google Scholar], [Indexed]
- Kumar R, Taha J, Greiner AL (1995) Herniation of the spinal cord: Case report. J Neurosurg 82: 131-136.
[Crossref], [Google Scholar], [Indexed]
- Selviaridis P, Balogiannis I, Foroglou N, Hatzisotiriou A, Patsalas I (2009) Spontaneous spinal cord herniation: Recurrence after 10 years. Spine J 9: 17-9.
[Crossref], [Google Scholar], [Indexed]
- Tekkok IH (2000) Spontaneous spinal cord herniation: Case report and review of the literature. Neurosurgery 46: 485-492.
[Crossref], [Google Scholar], [Indexed]
- DaSilva VR, Al-Gahtany M, Midha R, Sarma D, Cooper P (2003) Upper thoracic spinal cord herniation after traumatic nerve root avulsion: Case report and review of the literature. J Neurosurg 99: 306-309.
[Crossref], [Google Scholar], [Indexed]
- Chaichana KL, Sciubba DM, Li KW, Gokaslan ZL (2009) Surgical management of thoracic spinal cord herniation: Technical consideration. J Spinal Disord Tech 22: 67-72.
[Crossref], [Google Scholar], [Indexed]
- Kenez J, Barsi P, Varallyay G, Bobest M, Veres R (2002) MR investigation of spinal cord herniation in the thoracic spine. Ideggyogy Sz 55: 168-172.
[Crossref], [Google Scholar], [Indexed]
- Kwong Y, Jakanani G, Rao N, Fang CSJ (2010) MRI findings in herniation of the spinal cord. J Radiol Case Rep 4: 1-5.
[Crossref], [Google Scholar], [Indexed]
- Grewal SS, Pirris SM, Vibhute PG, Gupta V (2015) Identification of arachnoid web with a relatively novel magnetic resonance imaging technique. Spine J 15: 554–555.
[Crossref], [Google Scholar], [Indexed]
- Sharma P, Soin P, Elbanan M, Kochar PS (2019) Understanding Idiopathic Spinal Cord Herniation – A Comprehensive Review of Imaging and Literature. J Clin Imaging Sci 9: 1–4.
[Crossref], [Google Scholar], [Indexed]
- Imagama S, Matsuyama Y, Sakai Y, Nakamura H, Katayama Y et al. (2009) Image classification of idiopathic spinal cord herniation based on symptom severity and surgical outcome: A multicenter study. J Neurosurg Spine 11: 310-319.
[Crossref], [Google Scholar], [Indexed]
- Spissu A, Peltz MT, Matta G, Cannas A (2004) Traumatic transdural spinal cord herniation and the nuclear trail sign: Case report. Neurol Sci 25: 151–153.
[Crossref], [Google Scholar], [Indexed]
- Hawasli AH, Ray WZ, Wright NM (2014) symptomatic thoracic spinal cord herniation: case series and technical report. Neurosurgery 10: 498-504.
[Crossref], [Google Scholar], [Indexed]
- Borges LF, Zervas NT, Lehrich JR. (1995) Idiopathic spinal cord herniation: A treatable cause of the Brown-Sequard syndrome-case report. Neurosurgery 36: 1028–1033.
[Crossref], [Google Scholar], [Indexed]
- Ewald C, Hassler WE (2001) Spontaneous herniation of the thoracic spinal cord as the etiology of progressive Brown-Sequard syndrome. A description of 3 cases. Nervenarzt 72: 441–444.
[Crossref], [Google Scholar], [Indexed]
- Iunes EA, Barletta EA, Suzuki FS, Barba Belsuzarri TA, de Araújo Paz D, et al. (2020) Idiopathic Ventral Spinal Cord Herniation: Video Report and Systematic Review. World Neurosurg 139: 592–602.
[Crossref], [Google Scholar], [Indexed]
- Ulivieri S, Oliveri G, Petrini C, Elia FD, Cuneo GL et al. (2009) Thoracic spinal cord herniation: Case report and technical note. Neurologia I Neurochirurgia Polska 43: 86-89.
[Crossref], [Google Scholar], [Indexed]
- Arts MP, Lycklama a Nijeholt G, Wurzer JAL (2006) Surgical treatment of idiopathic transdural spinal cord herniation: A new technique to untether the spinal cord. Acta Neurochir 148: 1005–1009.
[Crossref], [Google Scholar], [Indexed]
- Ewald C, Kuhne D, Hassler WE (2000) Progressive spontaneous herniation of the thoracic spinal cord: Case report. Neurosurgery 46: 493-496.
[Crossref], [Google Scholar], [Indexed]
- Eguchi T, Yokota H, Nikaido Y, Nobayashi M, Nishioka T (2001) Spontaneous thoracic spinal cord herniation-case report. Neurol Med Chir 41: 508-512.
[Crossref], [Google Scholar], [Indexed]
- Pereira P, Duarte F, Lamas R, Vaz R (2001) Idiopathic spinal cord herniation: Case report and Literature Review. Acta Neurochir 143: 401-406.
[Crossref], [Google Scholar], [Indexed]
- Aizawa T, Sato T, Tanaka Y, Kotajima S, Sekiya M, et al. (2001) Idiopathic herniation of the thoracic spinal cord: Report of three cases. Spine 26: 488-491.
[Crossref], [Google Scholar], [Indexed]
- Berbel A, Porta EJ, Martinez SA, Perez MDA, Saiz-Diaz RA, et al. (2001) Idiopathic spinal cord herniation. Presentation of a new case and review of the literature. Rev Neurol 32: 54-57.
[Crossref], [Google Scholar], [Indexed]
- Adams RF, Anslow P (2001) The natural history of transdural herniation of the spinal cord: Case report. Neuroradiology 43: 383-387.
[Crossref], [Google Scholar], [Indexed]
- Nakagawa H, Kamimura M, Uchiyama S, Takahara K, Itsubo T, et al. (2003) Idiopathic spinal cord herniation associated with a large erosive bone defect: A case report and review of the literature. J Spinal Disord Tech 16: 299-305.
[Crossref], [Google Scholar], [Indexed]
- Sagiuchi T, Iida H, Tachibana S, Utsuki S, Tanaka R, et al. (2003) Idiopathic spinal cord herniation associated with calcified thoracic disc extrusion-case report. Neurol Med Chir 43: 364-368.
[Crossref], [Google Scholar], [Indexed]
- Najjar MW, Baeesa SS, Lingawi SS (2004) Idiopathic spinal cord herniation: A new theory of pathogenesis. Surg Neurol 62: 161-170.
[Crossref], [Google Scholar], [Indexed]
- Rivas JJ, la Lama A, Gonza LP, Ramos A, Zurdo M, et al. (2004) Spontaneous spinal cord herniation. Neurocirugia 15: 484-489.
[Crossref], [Google Scholar], [Indexed]
- Maruichi K, Hida K, Seki T, Iwasaki Y (2004) Idiopathic spinal cord herniation which extended remarkably up- and downward from dural defect: Case report. Neurological Surgery 32: 509-512.
[Crossref], [Google Scholar], [Indexed]
- Aquilina K, Nanra J S, Rawluk D (2004) Idiopathic spinal cord hernia. Ir Med J 97: 115-6.
[Crossref], [Google Scholar], [Indexed]
- Ferre JC, Carsin-Nicol B, Hamlat A, Carsin M, Morandi X (2005) MR imaging features of idiopathic thoracic spinal cord herniations using combined 3D-fiesta and 2D-PC Cine techniques. J Neuroradiol 32: 125-130.
[Crossref], [Google Scholar], [Indexed]
- Darbar A, Krishnamurthy S, Holsapple JW, Hodge CJ (2006) Ventral thoracic spinal cord herniation: Frequently misdiagnosed entity. Spine 31: 600-605.
[Crossref], [Google Scholar], [Indexed]
- Morley S, Naidoo P, Robertson A, Chong W (2006) Thoracic ventral dural defect: Idiopathic spinal cord herniation. Australas Radiol 50(2): 168–70.
[Crossref], [Google Scholar], [Indexed]
- Inoue A, Kohno K, Takeda T, Okuda B, Takechi A, et al. (2006) A case of high-aged idiopathic spinal cord herniation from dural defect. Neurological Surg 34: 627-631.
[Crossref], [Google Scholar], [Indexed]
- Saito A, Takahashi T, Sato S, Kumabe T, Tominaga T (2006) Modified surgical technique for the treatment of idiopathic spinal cord herniation. Minim Invasive Neurosurg 49: 120-123.
[Crossref], [Google Scholar], [Indexed]
- Bandai H, Ohara Y, Dei F, Mitsuoka H, Bando K (2006) A case of idiopathic thoracic spinal cord herniation. Brain and Nerve 58: 893-897.
[Crossref], [Google Scholar], [Indexed]
- Alkan O, Kizilkilic O, Goksel BK, Yildirim T, Sarica FB (2008) Ventral thoracic spinal cord herniation: A commonly misdiagnosed and treatable cause of myelopathy. Neuroradiol J 21: 563-567.
[Crossref], [Google Scholar], [Indexed]
- Ghostine S, Baron EM, Perri B, Jacobson P, Morsette D et al. (2009) Thoracic cord herniation through a dural defect: Description of a case and review of the literature. Surg Neurol 71: 362-6.
[Crossref], [Google Scholar], [Indexed]
- Senturk S, Guzel A, Guzel E (2008) Atypical clinical presentation of idiophatic thoracic spinal cord herniation. Spine 33: 474-477.
[Crossref], [Google Scholar], [Indexed]
- Sasani M, Ozer AF, Vural M, Sarioglu AC (2009) Idiopathic spinal cord herniation: Case report and review of the literature. J Spinal Cord Med 32: 86-94.
[Crossref], [Google Scholar], [Indexed]
- Nakamura M, Fujiyoshi K, Tsuji O, Watanabe K, Tsuji T, et al. (2011) Long-term surgical outcomes of idiopathic spinal cord herniation. J Orthop Sci 16: 347-351.
[Crossref], [Google Scholar], [Indexed]
- Liu Z, Wang WJ, Sun C, Zhu ZZ, Qiu Y (2011) Thoracic spinal cord herniation in a patient with long-standing ankylosing spondylitis. Eur Spine J 20: 222-226.
[Crossref], [Google Scholar], [Indexed]
- Akutsu H, Takada T, Nakai K, Tsuda K, Sakane M, et al. (2012) Surgical technique for idiopathic spinal cord herniation: The Hammock method. Technical note. Neurol Med Chir 52: 238-242.
[Crossref], [Google Scholar], [Indexed]
- Moriyama T, Tachibana T, Maruo K, Inoue S, Okada F et al. (2013) Postoperative spinal cord herniation with pseudomeningocele in the cervical spine: A case report. Spine J 13: 43-45.
[Crossref], [Google Scholar], [Indexed]
- Krishnan P, Kartikueyan R, Chowdhury D, Saha M (2013) Ventral herniation of the dorsal spinal cord: A rare cause of myelopathy. Neurol India 61: 453-454.
[Crossref], [Google Scholar], [Indexed]
- Yamamoto N, Katoh S, Higashino K, Sairyo K (2014) Idiopathic spinal cord herniation with duplicated dura mater and dorsal subarachnoid septum. Report of a case and review of the literature. Int J Spine Surg 8: 29.
[Crossref], [Google Scholar], [Indexed]
- De Souza RB, De Aguiar GB, Daniel JW, Veiga JCE (2014) The pathophysiology, classification, treatment, and prognosis of a spontaneous thoracic spinal cord herniation: A case study with literature review. Surg Neurol Int 5: 564–566.
[Crossref], [Google Scholar], [Indexed]
- Lui J, Sayal P, Choi D (2018) Spinal cord suspension using dentate ligament hitch stitches: A novel technique for the repair of ventral spinal cord herniation. Oper Neurosurg 14: 252-258.
[Crossref], [Google Scholar], [Indexed]
- Bartels RHMA, Brunner H, Hosman A, van Alfen N, Grotenhuis JA (2017) The pathogenesis of ventral idiopathic herniation of the spinal cord: a hypothesis based on the review of the literature. Front Neurol 8: 476.
[Crossref], [Google Scholar], [Indexed]
- Delgado-Lopez PD, Gil-Polo C, Martin-Velasco V, Martin-Alonso J, Galacho-Harriero AM, et al. (2017) Spinal cord herniation repair with microstaples: Case report. J Neurosurg Spine 26: 384-387.
[Crossref], [Google Scholar], [Indexed]
- Shimizu S, Kobayashi Y, Oka H, Kumabe T (2017) Idiopathic spinal cord herniation: Consideration of its pathogenesis based on the histopathology of the dura mater. Eur Spine J 28: 298-305.
[Crossref], [Google Scholar], [Indexed]
- Ghali MGZ, Srinivasan VM, Rao VY, Omeis I (2018) Idiopathic thoracic spinal cord herniation. J Clin Neurosci 51: 1-5.
[Crossref], [Google Scholar], [Indexed]
- Bartels RHMA, Kusters B, Brunner H, Hosman AJF, Van Alfen, N et al. (2018) Pathogenesis of idiopathic ventral herniation of spinal cord: Neuropathologic analysis. World Neurosurg 114: 30-33.
[Crossref], [Google Scholar], [Indexed]
- Herring EZ, Shin JH, Nagel SJ, Krishnaney AA (2019) Novel strategy of ventral dural repair for idiopathic thoracic spinal cord herniation: Report of outcomes and review of techniques. Oper Neurosurg 17: 21-31.
[Crossref], [Google Scholar], [Indexed]
- Neale N, Ramayya A, Welch W (2019) Surgical management of idiopathic thoracic spinal cord herniation. World Neurosurg 129: 81-84.
[Crossref], [Google Scholar], [Indexed]
- Finneran MM, Schaible K (2020) Ventral herniation of the cervical cord after single-level corpectomy. World Neurosurg 136: 12-16.
[Crossref], [Google Scholar], [Indexed]
- Aljuboori Z, Boakye M (2020) Rare dorsal thoracic arachnoid web mimics spinal cord herniation on imaging. Surg Neurol Int 11: 66.
[Crossref], [Google Scholar], [Indexed]
- Regensburger M, Schlachetzki JCM, Klekamp J, Doerfler A, Winkler J. (2020) Long-term course of anterior spinal cord herniation presenting with an upper motor neuron syndrome: Case report illustrating diagnostic and therapeutic implications. BMC Neurol 20: 1-6.
[Crossref], [Google Scholar], [Indexed]
- Diaz A, Burks SS, Fisher R, Levi AD (2021) Posterior surgical approach for ventral cervical spinal cord herniation: 2-dimensional operative video. Oper Neurosurg 20: 215-216.
[Crossref], [Google Scholar], [Indexed]
- Teng KX, Dimou J (2021) Delayed cord tethering post-ventral dural repair of idiopathic thoracic cord herniation. J Clin Neurosci 88: 1-4.
[Crossref], [Google Scholar], [Indexed]
- Wilson TA, Promod Kumar RP, Omosor E (2021) Thoracic ventral spinal cord herniation with progressive myelopathy–A case report and review of the literature. Surg Neurol Int 12: 382.
[Crossref], [Google Scholar], [Indexed]
- Parmar H, Park P, Brahma B, Gandhi D (2008) Imaging of idiopathic spinal cord herniation. Radiographics 28: 511-518.
[Crossref], [Google Scholar], [Indexed]
- Dunn V, Smoker W R, Menezes AH (1987) Transdural herniation of the cervical spinal cord as a complication of a broken fracture-fixation wire. Am J Neuroradiol 8: 724-726.
[Crossref], [Google Scholar], [Indexed]
- Burres KP, Conley FK (1978) Progressive neurological dysfunction secondary to postoperative cervical pseudomeningocele in a C-4 quadriplegic. Case report. J Neurosurg 48: 289-291.
[Crossref], [Google Scholar], [Indexed]
- Cobb C, Ehni G (1973) Herniation of the spinal cord into an iatrogenic meningocele. Case report. J Neurosurg 39: 533-536.
[Crossref], [Google Scholar], [Indexed]
- Yokota H, Yokoyama K, Noguchi H, Uchiyama Y (2007) Spinal cord herniation into associated pseudomeningocele after brachial plexus avulsion injury: Case report. Neurosurgery 60: 205.
[Crossref], [Google Scholar], [Indexed]
- Tanaka M, Ikuma H, Nakanishi K, Sugimoto Y, Misawa H, et al. (2008) Spinal cord herniation into pseudomeningocele after traumatic nerve root avulsion: Case report and review of the literature. Eur Spine J 17: 263-266.
[Crossref], [Google Scholar], [Indexed]
- Ijiri K, Hida K, Yano S, Komiya S, Iwasaki Y (2009) Traumatic spinal-cord herniation associated with pseudomeningocele after lower-thoracic nerve-root avulsion. Spinal Cord 47: 829-831.
[Crossref], [Google Scholar], [Indexed]
- Watters MR, Stears JC, Osborn AG, Turner GE, Burton BS, et al. (1998) Transdural spinal cord herniation: Imaging and clinical spectra. Am J Neuroradiol 19: 1337-1344.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences