Pulmonary Function and Quality of Life in Pediatric Patients with Duchenne Muscular Dystrophy
Isabel C.S. Ferreira*, Letícia R. Machado, Diego D. Brandenburg, Paulo J.C. Maróstica
1Universidade Federal do Rio Grande do Sul. Programa de Pós-Graduação em Saúde da Criança e do Adolescente. Ramiro Barcelos, 2400/220. Porto Alegre. Rio Grande do Sul. Brazil
2Hospital de Clínicas de Porto Alegre. Serviço de Pediatria. Unidade de Pneumologia Infantil. Ramiro Barcelos, 2350. Porto Alegre. Rio Grande do Sul. Brazil
- Corresponding Author:
- Isabel Cristina Schütz Ferreira
Universidade Federal do Rio Grande do Sul.
Programa de Pós-Graduação em Saúde da
Criança e do Adolescente. Ramiro Barcelos
2400/220. Porto Alegre. Rio Grande do Sul. Brazil.
Tel: +5551992430010
E-mail: isabelcsf.pediatria@gmail.com
Received Date: September 29, 2020; Accepted Date: October 16, 2020; Published Date: October 23, 2020
Citation: Ferreira ICS, Machado LR, Brandenburg DD, Maróstica PJC (2020) Pulmonary Function and Quality of Life in Pediatric Patients with Duchenne Muscular Dystrophy. J Rare Disord Diagn Ther Vol.6 No.5:10 doi:10.36648/2380-7245.6.5.208
Copyright: © 2020 Ferreira ICS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A drop of 20-25% in vital capacity from sitting to lying down position was associated with diaphragmatic weakness, in some studies in Duchenne muscular dystrophy. Also, there are few studies about the quality of life in these patients. We aimed to provide a better understanding of pulmonary function and quality of life of Duchenne muscular dystrophy patients and to evaluate the significance of the vital capacity drop from sitting to lying position. This was a cross-sectional study, with Duchenne muscular dystrophy patients, aged 6 to 18 years. A total of 14 patients were included in the study. There was a positive correlation between seated vital capacity z-score and maximal expiratory pressures (r=0.78; p<0.01). There was a negative correlation between lying vital capacity z-score, age (r=- 0.7; p<0.01) and partial pressure of carbon dioxide (r=-0.57; p<0.05). The mean drop in vital capacity from seated to lying was -8.5 ± 9.02%. The vital capacity drop did not show a correlation with the analyzed parameters. The quality of life questionnaire means score was 55.02 ± 10.03. Patients had low vital capacity and maximal expiratory pressure results. Adding a second spirometric maneuver in lying position did not add much in terms of previewing diaphragmatic muscular compromise.
Keywords
Duchenne Muscular Dystrophy; Spirometry; Vital Capacity; Diaphragmatic Weakness; Quality of Life
Introduction
Duchenne Muscular Dystrophy (DMD) is the most common fatal neuromuscular disease in the pediatric population. It is an X-linked recessive genetic disease, which affects 1 in 3300-7000 live births. It is caused by a mutation of the dystrophin gene, mainly deletions [1-3]. The disease is characterized by a progressive loss of muscle strength, being ventilatory failure as the main cause of death, even though life expectancy has improved in the latest decades mainly as a result of better ventilatory management. The diagnosis is based on clinical findings and should be confirmed by genetic analysis or muscle biopsy [4-7]. It is well established that patients should be followed with spirometry, at least once a year, but there is still no objective data on cut-off points that should determine interventions and most guidelines are based on expert consensus. The diaphragm is a muscle that produces a great part of the total inhalation during forced inspiration. As it occurs with other muscles in DMD, the diaphragm weakness probably gets worse with aging. Its weakness is correlated with orthopnea and maximal inspiratory pressure (MIP) reduced and can cause respiratory failure [8,9]. A study by Fromageot et al. found that a drop in vital capacity (VC) greater than 20-25% in changing the patient from sitting to lying down position was able to detect diaphragmatic weakness with sensitivity and specificity of 79% and 90%, respectively, taking as reference invasive diaphragmatic indexes. Won et al. followed longitudinally postural vital capacity (VC) difference and MIP of DMD patients, as an indirect index of diaphragmatic weakness. Unexpectedly both MIP and postural VC difference decreased with age, showing that VC postural difference paralleled diaphragmatic strength loss in DMD, when an inverse correlation would be expected [9,10]. To diagnosis diaphragmatic weakness early could allow better management of respiratory failure, having an impact on the quality of life and life expectancy. In the context of chronic and progressive disease, DMD may have an impact on patients and their family quality of life. When assessing the quality of life, clinicians should pay attention to both the biological and psychosocial aspects of the disease [11,12]. There are few studies about quality of life in DMD. To provide a better understanding of pulmonary function and quality of life of DMD patients and to evaluate the significance of the VC drop in the sitting and lying position change we conducted this study.
Patients and Methods
Study design and patients
This was a cross-sectional study that included children diagnosed with DMD by clinical findings and confirmed by a genetic test, aged 6-18 years, who attended the pediatric pulmonology outpatient clinic at Hospital de Clínicas de Porto Alegre (HCPA), Rio Grande do Sul, Brazil, between May 2019 and January 2020. All the patients were invited to participate in the study. The patients that were unable to perform spirometry maneuvers were excluded from the study. The study was reviewed and approved by the Ethical Review Board of HCPA. Each legal patient representative gave informed consent before entering the study. Clinical data were collected from medical records, including corticosteroid length of use, comorbidities, weight and height, left ventricular ejection fraction (LVEF) at echocardiogram, maximal respiratory pressures and PCO2 in blood gas analyses.
Spirometry
Spirometry was performed in the pulmonary function laboratory, following guidelines and acceptability criteria of the American Thoracic Society (ATS) statement. There was a thirty-minute interval between the sitting and lying spirometry. The spirometer used was IFV Vyntus Spiro, Jaeger and the software was SentrySuit, Version 2.11. The spirometry used for our study was the most recent one performed by the patient at the time of data collection, not longer than one month before. The prediction equations used were those from the Global Lung Function Initiative (GLI) network. All patients had their weight and height or wingspan measured before the exam. Wingspan was used for wheelchair dependent patients. The used scale brand was Filizola, manufactured in São Paulo, with a 100g precision. The used anthropometer brand was Sanny, ES 2030, with a 0.1cm precision.
Quality of life questionnaire
The quality of life questionnaire was the CHQ50-PF and it was answered by the patients’ legal guardians. The CHQ50-PF quality of life questionnaire is already validated for Brazilian children. It is composed of 50 questions and it is answered by parents or guardians. The questionnaire addresses physical and psychosocial aspects, with the following domains: global health, physical functioning, social limitations – emotional, social limitations – physical, bodily pain, behavior, global behavior, mental health, self-esteem, general health perceptions, change in health, parental impact – emotional, parental impact – time, family activities and family cohesion. Each domain scores from 0-100, being the final score the mean of all the domain scores, and the higher the score, the better the quality of life [13].
Measures
A drop in the VC after a position change from sitting to lying was the primary outcome measured. Secondary outcomes were CHQ50-PF score and correlations between VC, maximal respiratory pressures, age, left ventricular ejection fraction. We also analyzed clinical characteristics such as comorbidities, steroid length of use, physiotherapy adherence and age that patients became non ambulant.
Statistical analyses
Data were analyzed with the SPSS v.20.0. Categorical variables were described by frequency and percentage. The symmetry of the continuous variables was verified using the Shapiro Wilk test. Quantitative variables were described by means and standard deviations. To assess the association between quantitative variables, Pearson’s correlation coefficient was used. A significance level of 5% was considered for the established comparisons.
Results
Between May 2019 and January 2020, all the 17 patients with DMD followed at the pediatric pulmonology outpatient clinic of Hospital de Clínicas de Porto Alegre were invited to participate in the study. All the 17 patients were included, but 3 of them were not able to perform the spirometry maneuvers properly and were excluded, remaining a total of 14 patients. All variables analyzed were normally distributed. The mean age at diagnosis was 6 ± 2.08 years. Ten (71.4%) of the 14 patients were restricted to a wheelchair. The mean age to become non ambulant was 9.4 ± 1.17 years. The mean age at the time of the analyzed spirometry was 11.92 ± 3.24 years, the body mass index (BMI) mean was 23.26 ± 4.79 kg/m2, the BMI z-score mean was 1.30 ± 0.84. The mean left ventricular ejection fraction (LVEF) was 63.94 ± 9.31%. Most patients (85.7%) did not have any history of asthma, 3 (21.4%) were being followed by a speech therapist because of dysphagia. Concerning respiratory physiotherapy, 2 (14.3%) patients did not perform any, 2 (14.3%) once a week, 7 (50%) twice a week, 1 (7.1%) three times a week, 1 (7.1%) five times a week and 1 (7.1%) seven times a week. Of the 14 patients, three of them had not used corticosteroid; the mean length of use of the rest of the patients was 30.6 ± 25.20 months. The genetic analysis was made initially with MLPA and a mutation within DND gene was found in 12 (85.7%) patients. Two patients are waiting for the Sanger sequencing. The description of genetic analysis is shown in Table 1.
| Patients | Mutation | Clinical data |
|---|---|---|
| Patient 1 | Exons 48, 50, 51 and 52 deletion | Age at diagnosis: 4 yrs. old. Non ambulant at 10 yrs. old. Used corticosteroid for 5 months. |
| Age at spirometry: 12 yrs. old. | ||
| MIP: 5.95. | ||
| MEP: 3.24. | ||
| Z-score seated VC: - 1.55. | ||
| Z-score seated FEV1: -1.43. | ||
| Seated Tiff: 87. Z-score lying VC: -3.12. | ||
| Z-score lying FEV1: -2.90. | ||
| Lying Tiff: 87. | ||
| Drop VC from seated to lying: 21.53%. | ||
| Patient 3 | Exons 50, 51 and 52 deletion | Age at diagnosis: 7 yrs. old. Non ambulant at 10 yrs. old. Used corticosteroid for 53 months. |
| Age at spirometry: 17 yrs. old. | ||
| MIP: 4.27. | ||
| MEP: 2.80. | ||
| Z-score seated VC: -5.31. | ||
| Z-score seated FEV1: -4.70. | ||
| Seated Tiff: 84. | ||
| Z-score lying VC: -5.79. | ||
| Z-score lying FEV1: -5.05. | ||
| Lying Tiff: 95. | ||
| Drop VC from seated to lying: 12.96%. | ||
| Patient 4 | Exons 45, 47, 48, 50, 51, 52 deletion | Age at diagnosis: 3 yrs. old. Non ambulant at 9 yrs. old. Used corticosteroid for 60 months. |
| Age at spirometry: 15 yrs. old. | ||
| MIP: 2.13. | ||
| MEP: 1.97. | ||
| Z-score seated VC: -7.62. | ||
| Z-score seated FEV 1: -6.42. | ||
| Seated Tiff: 98. | ||
| Z-score lying VC: -7.71. | ||
| Z-score lying FEV1: -6.50. | ||
| Lying Tiff: 89. | ||
| Drop VC from seated to lying: 6.67%. | ||
| Patient 5 | Exons 3, 4, 5, 6, 7 deletion | Age at diagnosis: 3 yrs. old. Still ambulant. Used corticosteroid for 80 months. Age at spirometry: 10 yrs. old. |
| MIP: 7.89. | ||
| MEP: 8.17. | ||
| Z-score seated VC: -0.49. | ||
| Z-score seated FEV 1: -0.50. | ||
| Seated Tiff: 84. | ||
| Z-score lying VC: -0.31. | ||
| Z-score lying FEV 1: -0.84. | ||
| Lying Tiff: 77. Did not have a drop in VC from seated to lying. | ||
| Patient | Mutation | Clinical data |
| Patient 6 | Exon 2 duplication | Age at diagnosis: 9 yrs. old. Non ambulant at 8 yrs. old. Used corticosteroid for 6 months. |
| Age at spirometry: 11 yrs. old. | ||
| MIP: 6.23. | ||
| MEP: 3.63. | ||
| Z-score seated VC: -3.48. | ||
| Z-score seated FEV 1: -3.73. | ||
| Seated Tiff: 80. | ||
| Z-score lying VC: -3.65. | ||
| Z-score lying FEV1: -4.07. | ||
| Lying Tiff: 77. | ||
| Drop VC from seated to lying: 2.47%. | ||
| Patient 7 | Exons 8 and 9 deletion | Age at diagnosis: 5 yrs. old. Still ambulant. Used corticosteroid for 28 months. |
| Age at spirometry: 9 yrs. old. | ||
| MIP: 4.31. | ||
| MEP: 8.89. | ||
| Z-score seated VC: 2.19. | ||
| Z-score seated FEV 1: 2.19. | ||
| Seated Tiff: 87. Z-score lying VC: 1.50. | ||
| Z-score lying FEV 1: 1.68. | ||
| Lying Tiff: 84. | ||
| Drop VC from seated to lying: 6.64%. | ||
| Patient 8 | Exons 46 to 55 deletion | Age at diagnosis: 5 yrs. old. Non ambulant at 9 yrs. old. Used corticosteroid for 21 months. |
| Age at spirometry: 14 yrs. old. | ||
| MIP: 1.91. | ||
| MEP: 2.35. Z-score seated VC: -5.57. |
||
| Z-score seated FEV 1: -4.70. | ||
| Seated Tiff: 87. | ||
| Z-score lying VC: -6.31. | ||
| Z-score lying FEV1: -5.32. | ||
| Lying Tiff: 95. | ||
| Drop VC from seated to lying: 21.43%. | ||
| Patient 9 | Exons 3 to 16 duplication | Age at diagnosis: 10 yrs. old. Non ambulant at 8 yrs. old. Did not use corticosteroid. |
| Age at spirometry: 12 yrs. old. | ||
| MIP: 4.00. | ||
| MEP: 2.87. | ||
| Z-score seated VC: -2.33. | ||
| Z-score seated FEV 1: -1.25. | ||
| Seated Tiff: 97. | ||
| Z-score lying VC: -2.42. | ||
| Z-score lying FEV 1: -1.73. | ||
| Lying Tiff: 90. | ||
| Drop VC from seated to lying: 1.40%. | ||
| Patient | Mutation | Clinical data |
| Patient 10 | Exons 5 to 39 deletion | Age at diagnosis: 8 yrs. old. Non ambulant at 11 yrs. old. Used corticosteroid for 34 months. |
| Age at spirometry: 17 yrs. old. | ||
| MIP: 8.20. | ||
| MEP: 5.91. | ||
| Z-score seated VC: -2.45. | ||
| Z-score seated FEV 1: -2.19. | ||
| Seated Tiff: 88. | ||
| Z-score lying VC: -2.95. | ||
| Z-score lying FEV1: -2.73. | ||
| Lying Tiff: 84. | ||
| Drop VC from seated to lying: 7.90%. | ||
| Patient 12 | Exons 49 to 52 deletion | Age at diagnosis: 6 yrs. old. Still ambulant. Used corticosteroid for 11 months. |
| Age at spirometry: 7 yrs. old. | ||
| MIP: 5.70. | ||
| MEP: 5.04. | ||
| Z-score seated VC: -0.34. | ||
| Z-score seated FEV 1: 0.21. | ||
| Seated Tiff: 96. | ||
| Z-score lying VC: -1.03. | ||
| Z-score lying FEV 1: -0.83. | ||
| Lying Tiff: 92. | ||
| Drop VC from seated to lying: 9.02%. | ||
| Patient 13 | Exon 51 deletion | Age at diagnosis: 7 yrs. old. Non ambulant at 10 yrs. old. Using corticosteroid for 36 months. Age at spirometry: 10 yrs. old. |
| MIP: 7.46. | ||
| MEP: 7.59. | ||
| Z-score seated VC: -1.00. | ||
| Z-score seated FEV 1: -1.19. | ||
| Seated Tiff: 85. | ||
| Z-score lying VC: -1.81. | ||
| Z-score lying FEV1: -1.73. | ||
| Lying Tiff: 94. | ||
| Drop VC from seated to lying: 10.44%. | ||
| Patient 14 | Exons 46 and 47 deletion | Age at diagnosis: 6 yrs. old. Still ambulant. Using corticosteroid for 3 months. |
| Age at spirometry: 7 yrs. old. | ||
| MIP: 3.21. | ||
| MEP: 3.94. | ||
| Z-score seated VC: -0.46. | ||
| Z-score seated FEV 1: 0.35. | ||
| Seated Tiff: 93. | ||
| Z-score lying VC: -0.29. | ||
| Z-score lying FEV 1: 0.48. | ||
| Lying Tiff: 95. Did not have a drop in VC from seated to lying. |
Table 1 Mutations in the DMD gene of 12 patients and their clinical data.
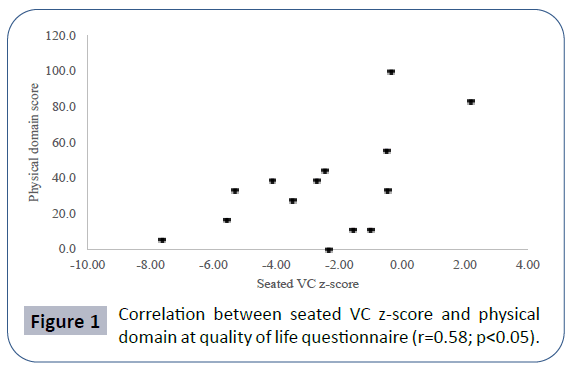
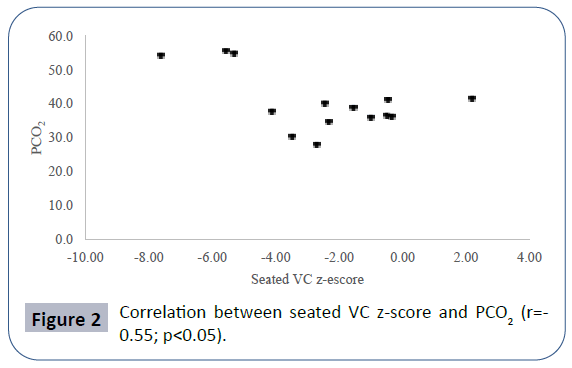
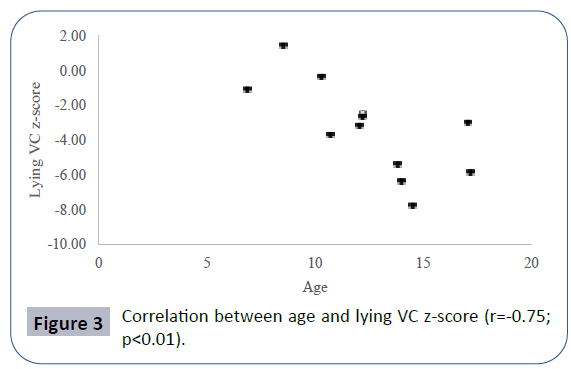
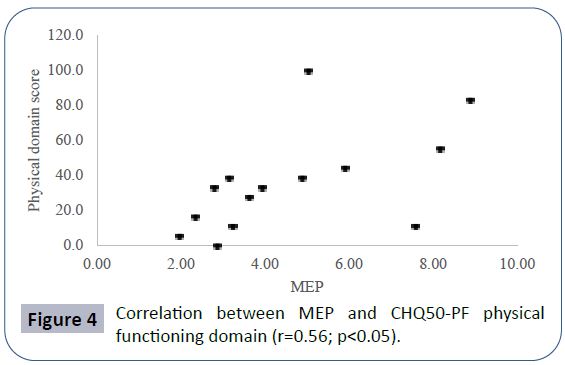
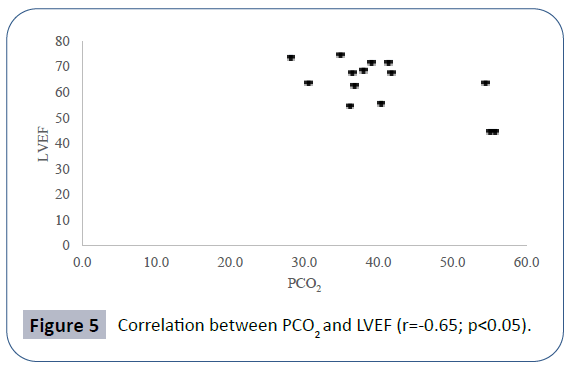
Pulmonary function data analyzed showed seated VC z-score mean -2.52 ± 2.56, lying VC z-score mean -2.99 ± 2.59, maximal inspiratory pressure (MIP) mean 4.83 ± 2.11 kPa, maximal expiratory pressure (MEP) mean 4.60 ± 2.25 kPa. Mean PCO2 in blood gas analyses was 40.66 ± 8.69 mmHg. There was a positive correlation between seated VC z-score and MEP (r=0.78; p<0.01), physical domain at quality of life questionnaire (r=0.58; p<0.05) (Figure 1) and a negative correlation between seated VC z-score, age (r=-0.73; p<0.01) and PCO2 (r=-0.55;p<0.05) (Figure 2). There was also a negative correlation between lying VC z-score, age (r=-0.75; p<0.01) (Figure 3) and PCO2 (r=-0.75; p<0.05). Lying VC z-score had a positive correlation with the physical domain (r=0.57; p<0.05) and MEP (r=0.79; p< 0.01). MEP had a positive correlation with the CHQ50-PF physical functioning domain (r=0.56; p<0.05) (Figure 4). The mean drop in the VC in changing from seated to lying was -8.5% ( ± 9.02). The greatest drop was 25% and it occurred in one patient. This patient was 14 years old, had not used corticosteroid and has been non-ambulant since the age of 10. Two patients had a drop of 21% and the rest of them had it below 20%. The VC drop did not show a correlation with any of the analyzed parameters. PCO2 showed a negative correlation with LVEF (r=-0.65;p<0.05) (Figure 5), CHQ50-PF health perception domain (r=-0.61, p<0.05) and CHQ50-PF change in health domain (r=-0.58, p<0.05). The quality of life questionnaire mean score was 55.02 ( ± 10.03), and it had a positive correlation with all the domains that involved physical aspects, such as the physical functioning (r=0.60; p<0.05), social limitation secondary to physical limitation (r=0.57;p<0.05) and also with the domain that evaluates the change in health in the last year (r=0.60; p<0.05).
Discussion
It was clear in our results that, as age advances, muscle weakness worsens and it has an impact on maximum respiratory pressures and, consequently, on the pulmonary ventilatory function. Patients had low VC results, in both lying and sitting positions, as well as in maximum inspiratory and expiratory pressures, as would be expected in DMD patients who have a restrictive ventilatory compromise secondary to diaphragmatic strength loss and spinal deformity. Most of the patients were restricted to a wheelchair before the second decade of life, in agreement with the literature data [14]. Adding a second spirometric maneuver in lying position did not add much in terms of previewing muscular compromise, although correlation coefficients were a little higher than those in sitting position. In a previous study, a 20-25% drop in the VC in spirometry when changing the patient from seated to lying was associated with diaphragmatic weakness [10-14]. One explanation could be that the enrolled patients had still not had enough diaphragmatic strength loss to be detected by spirometry in the present study, differently from other studies that included patients with more advanced disease. Also, a small sample of patients studied could in part explain these nonsignificant findings. In this study, we found other data parameters that are also important in the patients’ follow-up, such as PCO2. It is already known that PCO2 is one of the markers that must be monitored, since with the progression of the disease patients tend to hypoventilate. We found a negative correlation between the vital capacity z-score (both seated and lying) and PCO2, which shows the hypoventilation as the disease progresses. Also, we found a negative correlation between PCO2 and LVEF, which agrees with the multisystemic involvement (lung and heart) of the disease.
Conclusion
The impact of DMD on the quality of life of patients and their families was evident especially concerning the physical functioning domain, as evidenced by low scores in the quality of life questionnaire. It is well known that physical restriction is part of the spectrum of the disease and interventions to improve quality of life and social adjustment of patients are important issues to be dealt with. The main limitations of this study were the small number of evaluated patients and its cross-sectional design. There are few studies in DMD pediatric patients dealing both with the respiratory compromise and quality of life. So, the strength of our study was that we evaluated both these issues in DMD pediatric patients. Despite the small number of studied subjects, strong correlations and concordant results with other studies were found. Longitudinal studies of pulmonary function and quality of life with a greater number of pediatric patients with DMD are necessary to find out clinical signs and other determinants that can show early diaphragmatic weakness and for better evaluation of their quality of life during the disease progression. It is possible that following longitudinally such patients, spirometry variations between seated and lying positions could prove to be sensitive predictors of disease progression.
References
- Emery AE (1991) Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord 1: 19-29.
- Finder J, Mayer OH, Sheehan D, Sawnani H, Abresch RT, et al. (2017) Pulmonary Endpoints in Duchenne Muscular Dystrophy. A Workshop Summary. Am J Respir Crit Care Med 196: 512-519.
- Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, et al. (2004) Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med 170: 456-465.
- Mah JK, Korngut L, Fiest KM, Dykeman J, Day LJ, et al. (2015) A Systematic Review and Meta-analysis on the Epidemiology of the Muscular Dystrophies. Can J Neurol Sci 43: 163-177.
- Lee HN, Sawnani H, Horn PS, Rybalsky I, Relucio L, et al. (2016) The Performance of the Upper Limb scores correlate with pulmonary function test measures and Egen Klassifikation scores in Duchenne muscular dystrophy. Neuromuscul Disord 26: 264-271.
- LoMauro A, D'Angelo MG, Aliverti A (2015) Assessment and management of respiratory function in patients with Duchenne muscular dystrophy: Current and emerging options. Ther Clin Risk Manag 11: 1475-1488.
- Ripamonti E, D'Angelo G (2018) Measurement of respiratory function decline in patients with Duchenne muscular dystrophy: A conjoint analysis. Neurodegener Dis Manag 8: 89-96.
- Laviola M, Priori R, D’Angelo MG, Aliverti A (2018) Assessment of diaphragmatic thickness by ultrasonography in Duchenne muscular dystrophy (DMD) patients. PLoS One 13: e0200582.
- Fromageot C, Lofaso F, Annane D, Falaize L, Lejaille M, et al. (2017) Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 82: 123-128.
- Won YH, Choi WA, Kim DH, Kang SW (2015) Postural vital capacity difference with aging in Duchenne muscular dystrophy. Muscle Nerve 52: 722-727.
- Houwen-Van Opstal SL, Jansen M, Van Alfen N, De Groot IJ (2014) Health-related quality of life and its relation to disease severity in boys with Duchenne muscular dystrophy: satisfied boys, worrying parents--a case-control study. J Child Neurol 29: 1486-1495.
- Souza JG, Pamponet MA, Souza TC, Pereira AR, Souza AG, et al. (2014) Tools used for evaluation of Brazilian children's quality of life. Rev Paul Pediatr 32: 272-278.
- HealthAct CHQ (2013) The CHQ Scoring and Interpretation Manual. Boston, MA, USA.
- Pedlow K, McDonough S, Lennon S, Kerr C, Bradbury I, et al. (2019) Assisted standing for Duchenne muscular dystrophy. Cochrane Database Syst Rev 10: CD011550.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences