Polygonum amplexicaule Extract: An Effective Herbal Cure to CCl4 Induced Liver Damage In Vivo
Faiza Maqsood, Tanvir Ibrahim, Ammad Ahmad Farooqi and Muhammad Sheeraz Ahmad
DOI10.21767/2380-7245.100057
Faiza Maqsood1, Tanvir Ibrahim2, Ammad Ahmad Farooqi3 and Muhammad Sheeraz Ahmad1*
1Department of Biochemistry, PMAS-Arid Agriculture University Rawalpindi, Pakistan
2National Institute of Health, NIH, Islamabad, Pakistan
3Institute of Biomedical and genetic Engineering (IBGE), Islamabad, Pakistan
- *Corresponding Author:
- Muhammad Sheeraz Ahmad
Department of Biochemistry
PMAS-Arid Agriculture University Rawalpindi, Pakistan
Tel: 92-333-5706221
E-mail: dr.sheeraz@uaar.edu.pk
Received Date: May 04, 2017; Accepted Date: May 09, 2017; Published Date: May 16, 2017
Citation: Maqsood F, Ibrahim T, Farooqi AA, et al. Polygonum amplexicaule Extract: An Effective Herbal Cure to CCl4 Induced Liver Damage In Vivo. J Rare Disord Diagn Ther. 2017, 3:4. doi: 10.21767/2380-7245.100057
Abstract
Introduction: Liver damages are mainly caused by toxic chemicals, alcohol, infections and autoimmune disorders. Polygonum amplexicaule is a member of the genus Polygonum having high antioxidant contents and it has traditional medicinal attributes in treatment of many ailments particularly liver damage.
Objectives: Keeping in view the medicinal importance of P. amplexicaule, present study was constructed for in-vivo hepatoprotective activity of its rhizome extract in albino mice.
Methods: BALB/C albino mice were selected for experimental assays and sorted in nine groups (group i-iii control groups, group iv-ix herb treated groups) with six mice in each group for six days treatment. Blood samples from anesthetized mice (20% chloroform) were collected by carotid artery puncture allowed to coagulate at room temperature for 30 min and then centrifuged at 3000 rpm for 8-10 min. The serum separated was preserved at -20°C for subsequent analysis of biochemical parameters. Animals were sacrificed under diethyl ether anaesthesia at fasting state, liver tissues were quickly excised and were stored in fixative sera (10% formalin), for histology.
Results: Results revealed that CCl4 had significantly reduced the body weight and increased the level of ALT, AST, ALP and plasma bilirubin compared to toxic control. The pre-treatment of CCl4-administrated mice with extracts of P. amplexicaule at concentration of 200 mg/kg induced a profound decrease (62-94.4%) in the elevated activities of ALT, AST, ALP and plasma bilirubin level.
Conclusion: P. amplexicaule extracts have hepatoprotective activity and it can be used as herbal medicine for use in food, medicines and healthcare products to cure liver damages.
Keywords
Polygonum amplexicaule; Autoimmune disorders; Bilirubin
Introduction
Carbon tetrachloride (CCl4) is non-polar organic solvent with sweet smell like ether that can be detected at low level [1]. It is clear, heavy, non-inflammable liquid and used for induction of liver cirrhosis in animal model [2]. CCl4 undergoes enzymatic activation in endoplasmic reticulum by cytochrome P-450 dependent mixed oxidase (CYP2E1) and breaks down into highly toxic trichloromethyl CCl3 and trichloromethyl peroxyl (CCl3O2) free radicals [3]. These free radicals combine with cellular proteins and lipids in aerobic conditions followed by chloromethylation and saturation, to induce lipid peroxidation [4,5].
Mammalian cells are equipped with both enzymatic and non-enzymatic antioxidant defences with different efficacies that protect cells against oxidative damage caused by wide range of toxins including CCl4 [6]. To prevent the damage caused by free radicals, organisms have endogenous protective antioxidants. Toxic oxygen metabolites are detoxified by catalyzing reactions of important enzymatic antioxidants like SOD, CAT and GSH-Px [7].
Although there is tremendous advancement in the field of medicine but to stimulate liver functions still no effective drug is available in the market to cure liver and help hepatic cells to regenerate [8]. As compared to synthetic medicines, herbal medicines are more popular due to a number of reasons [9] such as they have no toxic effects and no side effects [10] are less cost effective [11] and much more easily handy [12,13]. So medicinal plants having important antioxidants and hepatoprotective activities was used in experimental animal model to cure liver damage. Due to its highest toxic effects CCl4 is most widely used to induce toxicity in experimental models and used to study the hepatoprotective effects of synthetic and herbal drugs [2,4,5,8].
The genus Polygonum (Polygonaceace) having 150 species producing high contents of important secondary metabolites like flavonoids, phenolics, cardiac glycosides and terpenoids [14]. Polygonum amplexicaule (local name Masloon) having flowering season June to September is widely distributed in the North Pakistan having high antioxidant content. Locally P. amplexicaule is used for the treatment of liver damage, flue, fever, joint pain and gastrointestinal disorders and also other species to cure dysentery, heart problems, ulcer and menstruation [15].
Keeping in view the medicinal importance of P. amplexicaule, present study is constructed for determination of hepatoprotective activity of the medicinally important plant species rhizome extract on animal model.
Materials and Methods
Chemicals and reagents
All the chemicals and reagents used for these experiments are of analytical grade. Carbon tetrachloride, silymarin and olive oil were obtained from the drug control and traditional medicine division NIH, Islamabad. Diagnostic kits for ALT, AST, ALP and serum bilirubin estimation were also obtained from NIH, Islamabad.
Plant collection
Sample of Polygonum amplexicaule was collected from Murree hills and herbarium sheets were prepared, dried and then identified. Plant rhizome was used for this study. Whole work was done in National institute of health NIH, Islamabad.
Protocol for plant extraction
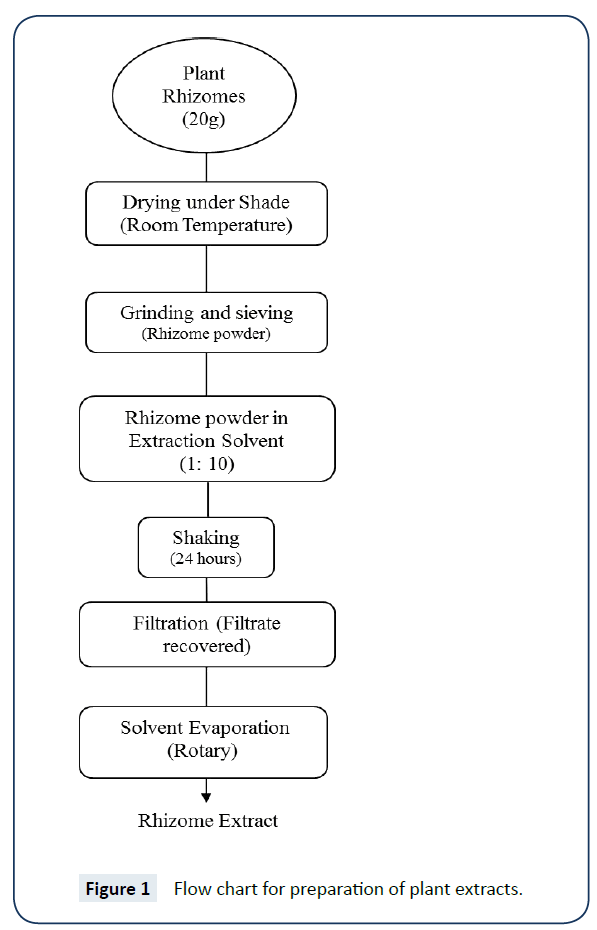
For preparation of plant extract, dried plant sample was griened, sived, Solution prepared, shaked and finally dried to get extract. A schematic procedure is given in flow chart extraction process (Figure 1).
Solution preparation
For extraction process solutions were made in polar solvents (distilled water, ethanol and methanol) in different ratios which are described in Table 1. For this purpose glassware like conical flask and cylinders were used. These glass wares were washed with distilled water and then sterilized. Sterilization process was done in an oven at 150°C for 15 min. This sterilized glassware’s were used for preparation of solutions.
| Plant | Solvent | Weight (g) | Volume (ml) |
|---|---|---|---|
| Polygonum amplexicaule | Methanol | 20 | 200 |
| Polygonum amplexicaule | Ethanol | 20 | 200 |
| Polygonum amplexicaule | Distilled Water | 20 | 200 |
Table 1: Amount of Plant material and volume of solvent for the preparation of extracts.
Respective amount of plant material were weighed with the help of electric weighing balance (Shimadzu) and volume of solvents like distilled water, ethanol and methanol was measured by using cylinders. Then plant materials were added into their respective type of solvent i.e., 20 g of Polygonum amplexicaule was added in 200 ml of ethanol, methanol and distilled water respectively. Conical flasks must be covered with rubber caps or aluminium foil [16]. Shaking of the solutions was done at 180 rpm at 36oC in Eyela shaking bath for 24 h. All samples were shaked 6 h daily and this process was completed in 4 days [16]. Solutions were filtered using Whatman filter paper of 42 cm pore size. Distilled water sample was filtered late as compared to methanolic and ethanolic samples [16].
Evaporation
After filtration, next step was to evaporate solvents from solution by using rotary vaccum evaporator. As the solution evaporates, it gets thick. Then that thick paste was transferred to falcon tubes. This thick paste must be in a dried form. For that purpose, these falcon tubes were placed in a water bath at 80°C. This process took 1-2 weeks. This dried form of plant extract was used for dose preparation [16].
Experimental animals
Healthy old Balb-C mice (28-40 g) of either sex were obtained from Animal house of NIH, Islamabad and were maintained on a standard laboratory diet. Mice were kept in standard poly-propylene cages at a 12-h cycle of light and dark. Room temperature at 24 ± 5°C and humidity maintained at 45-50%. All the chemicals used in this experiment were of the analytical grade, purchased from standard companies.
Toxicity studies
A study of acute toxicity was conducted according to the Organization for Economic Cooperation and Development (OECD) (OECD, 2001). A limited dose of 400 mg/kg of the aqueous, methanolic and ethanolic extracts of rhizome of Polygonum amplexicaule was used for the study.
Dose determination
Plant extract was dissolved in 0.9% normal saline solution. Calculations were as under:
For saline solution
Daily maximum dose consumption for each mouse was 100 μL. For six mice, 600 μL of saline solution was required. For six days, total dose administration was 3600 μL.
For 1 mice=100 μL;
For 6 mice=600 μL;
For 6 mice for 6 days=600 × 6=3600 μL;
Then, 3600 μL=3.6 ml.
Plant extract calculation
For 30 g of mice=400/1000 × 30=12 mg;
For 6 mice=12 × 6=72 mg;
For 6 days, 6 mice=72 × 6=432 mg.
Total 432 mg of methanolic, ethanolic and aqueous extract was weighed independently and transferred into separate falcon tubes of 15 ml. Then 3.6 ml of saline solution was added to these falcon tubes with the help of Apple syringes. Methanolic extracts were reluctant to dissolve into saline as compared to aqueous extracts therefore; sonicator was used for efficient dissolving. Sonication was done at maximum level for 30 min.
Dose Administration for Toxicity Studies
Two groups each containing six mice were sorted in two cages. Each mouse was delivered with 0.1 ml of dose daily for consecutive six days.
Carbon Tetrachloride Induced Hepatotoxicity
BALB-C albino mice were selected for experimental assays and sorted in groups of six mice in each cage and each group received six days treatment of;
Group I: (Negative control) mice received normal food and water.
Group II: (Induction control 25% CCl4, 1 ml/kg) received normal saline daily.
Group III: Treated with standard drug silymarin (100 mg/kg b.w).
Group IV-IX: Served as herb treated groups as below;
Group IV: Treated with P. amplexicaule (200 mg/kg b.w.) methanolic extract.
Group V: Treated with P. amplexicaule (300 mg/kg b.w.) methanolic extract.
Group VI: Treated with P. amplexicaule (200 mg/kg b.w.) ethanolic extract.
Group VII: Treated with P. amplexicaule (300 mg/kg b.w.) ethanolic extract.
Group VIII: Treated with P. amplexicaule (200 mg/kg b.w.) aqueous extract.
Group IX: Treated with P. amplexicaule (300 mg/kg b.w.) aqueous extract.
At the end of each treatment, mice were weighed and anesthetized with 20% chloroform. Blood samples from anesthetized mice were collected by carotid artery puncture allowed to coagulate at room temperature for half an hour. Blood samples were then centrifuged at 3000 rpm for 8-10 min. The serum separated was preserved at -20°C for subsequent analysis of biochemical parameters. Animals were sacrificed under diethyl ether anesthesia at fasting state, liver tissues were quickly excised and were stored in fixative sera (10% formalin), for histology.
Determination of body weight increase
Experimental animals were weighed before and after the onset of toxicity at the end of the trial and %age change in body weight were calculated for each animal using formula:
Change in weight (%)=[(final wt.-initial wt.)/initial wt.] × 100
Biochemical Assays
Determination of Alanine-Aminotransferase (ALT) activity
ALT (E.C.:2.6.1.2) activity was determined according to methods described by Reitman and Frankel [17] using GPT-ALT LR reagent kit purchased from Merck, Germany.
Principle
Alanine+α-ketoglutarate  Pyruvate+Glutamate Pyruvate formed reacts with 2,4-dinitrophenyl hydrazine to form pyruvate hydrazone complex which can be determined spectrophotometrically at 505 nm. Enzyme activity was obtained from standard curve by using absorbance of test samples.
Pyruvate+Glutamate Pyruvate formed reacts with 2,4-dinitrophenyl hydrazine to form pyruvate hydrazone complex which can be determined spectrophotometrically at 505 nm. Enzyme activity was obtained from standard curve by using absorbance of test samples.
Determination of Aspartate- Aminotransferase (AST) activity
AST (E.C.:2.6.1.1) activity on serum was determined as method described by Reitman and Frankel [17] having AST reagent kit obtained from Merck, Germany.
Principle
L-aspartate+α-oxaloglutarate  oxaloacetate+L-glutamate
oxaloacetate+L-glutamate
Oxaloacetate+NADH+H+ Malate+NAD+
Malate+NAD+
The rate of absorbance changing at λ=334 nm is directly proportional to AST activity which was determined from standard curve.
Determination of Alkaline Phosphatase (ALP) activity
Activity of alkaline phosphatase was determined according to the method of Res [18] by using reagent kit purchased from Merck, Germany.
Principle
P-nitrophenylophosphate+H2O 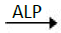 p-nitrophenol+phosphate
p-nitrophenol+phosphate
The rate of p-nitrophenol formation is directly proportional to ALP activity which was determined from standard curve.
Determination of plasma bilirubin (Total)
Bilirubin level determination was done by the method described by Jendrassik and Grof [19] by using reagent kit purchased from Merck, Germany.
Principle
Bilirubin+diazotized Sulfanic acid  Azobilirubin
Azobilirubin
Normal saline is used as an accelerator instead of caffeine, total bilirubin content couples with sulfanilic acid to form a red azobilirubin dye. The color intensity of the dye is proportional to the bilirubin concentration for direct bilirubin determination.
Statistical Analysis
The mean ± SD was calculated for each parameter. Data for total variation was estimated by one way Analysis of Variance (ANOVA), followed by student’s ‘t’ test. P<0.001 was considered as statistically significant when compared to control group. The percentage of the protection is calculated as reported by Rawat et al. [20].

Results and Discussion
Hepatoprotective studies
This study was conducted to evaluate the hepatoprotective activity of Polygonum amplexicaule extract against carbon tetrachloride-induced toxicity in mice. In this study, hepatoprotective activity of aqueous, methanolic and ethanolic extracts of P. amplexicaule was examined at different doses.
Acute toxicity test of extracts of Polygonum amplexicaule rhizome
An experiment was performed to check toxicity level of plant extracts and results indicated that animals used for assessment of acute toxicity, normally gained their body weight as shown in Table 2. None of the mice died or showed any sign of toxicity at limited dose of 400 mg/kg in the first 48 h and no apparent evidence of toxicity was noted during the period of observation. This is evident from the results that any extract of Polygonum amplexicaule has no toxic or lethal effects up to dose of 400 mg/kg.
| S. No. | Plant extract | Body weight | ||
|---|---|---|---|---|
| Before experiment (g) | After experiment (g) | % age change | ||
| 1 | Polygonum amplexicaule methanolic extract | 33.81 ± 1.3 | 35.49 ± 1.55 | 4.96 ± 0.9 |
| 2 | Polygonum amplexicaule ethanolic extract | 40.63 ± 1.03 | 42.67 ± 1.05 | 4.99 ± 0.2 |
| 3 | Polygonum amplexicaule aqueous extract | 37.18 ± 1.46 | 38.58 ± 1.83 | 3.77 ± 0.05 |
Values expressed as means ± S.D (n=6)
Table 2: Acute toxicity study of Polygonum amplexicaule rhizome.
Results indicated a negative effect of carbon tetrachloride on body weight and positive body weight gain in those CCL4- administered mice pretreated with standard drug (Silymarin) and different extracts of P. amplexicaule rhizome. Results are represented in Table 3.
| Groups | Body Weight (g) | ||
|---|---|---|---|
| Before Experiment | After Experiment | Change in Weigh (%)t | |
| Normal control | 20.33 ± 0.25 | 24.43 ± 0.28 | 20.16 ± 2.40 |
| Solvent control Normal saline (10 ml/kg) | 20.14 ± 0.52 | 22.72 ± 0.24 | 12.33 ± 1.82 |
| Induction control (25% CCl4, 1 ml/kg) | 20.55 ± 0.23* | 18.98 ± 0.50* | -8.27 ± 1.10* |
| Drug control (Silymarin 100 mg/kg)+CCl4 | 23.51 ± 0.23 λ | 26.07 ± 0.16 λ | 10.88 ± 1.12 λ |
| Polygonum amplexicaule methanolic extract (200 mg/kg)+CCl4 | 25.29 ± 0.27 α | 28.57 ± 0.31 α | 12.96 ± 1.13α |
| Polygonum amplexicaule methanolic extract (300 mg/kg)+CCl4 | 20.41 ± 0.23 | 21.84 ± 0.21 | 7.00 ± 0.13 |
| Polygonum amplexicaule ethanolic extract (200 mg/kg)+CCl4 | 25.72 ± 0.61 α | 27.80 ± 0.29 α | 8.08 ± 0.59 α |
| Polygonum amplexicaule ethanolic extract (300 mg/kg)+CCl4 | 24.99 ± 0.30 | 26.66 ± 0.41 | 6.68 ± 0.12 |
| Polygonum amplexicaule aqueous extract (200 mg/kg)+CCl4 | 21.91 ± 0.92 βλ | 25.49 ± 1.08βλ | 16.3 ± 1.32βλ |
| Polygonum amplexicaule aqueous extract (300 mg/kg)+CCl4 | 28.11 ± 0.91 | 29.89 ± 0.81 | 6.37 ± 0.50 |
Data are expressed as mean ± S.D (n=6)
Values differ significantly compared with normal control: *P<0.05
Values significantly different compared to CCl4-administered control: αP<0.05, λP<0.01
Values significant varied comparable to standard drug: βP<0.001
Table 3: Effects of Polygonum amplexicaule on body weight gain in CCl4 administered mice.
CCl4-administered mice exhibited a detectable decrease in weight as compared with normal mice (P<0.05). This decrease in the body weight may be due to alteration in nutrient absorption as loss of food intake and metabolic utilization after pretreatment with CCl4 as indicated by many publications [21,22].
The pretreatment of CCL4 administered mice with standard silymarin and different concentrations of P. amplexicaule extracts exerted a significant raise in the body weight as compared to CCl4 administered control group. P<0.01 clearly revealed that results are highly significant (12.96 ± 1.13%) and (16.3 ± 1.12%) in case of methanolic and aqueous extracts of P. amplexicaule at concentration of 200 mg/kg b.w as compared to CCl4 administered control (-8.27 ± 1.10%).
Significant increase in the body weight might be due to high flavonoid and phenolic constituent which may improve the nutritional and healthy states leading to an enhanced body weight gain. The high flavonoid diet is reported for decrease in weight caused by CCl4 exposure Krishna et al. [21] and Singh et al. [23].
Effect of Plant Extract on Serum Enzyme Activities and Bilirubin Total Proteins
In the present study, toxic effect of CCl4 cause extensive damage to the liver, reflected by the elevation of serum ALT, AST, ALP as well as rise in plasma bilirubin levels.
ALT activity
ALT is the most reliable enzyme used to indicate hepatic damage to hepatocytes [24]. ALT is more selectively a liver parenchymal enzyme than AST. Data are showing the effect of methanolic, ethanolic and aqueous extracts of P. amplexicaule on serum ALT level in CCl4-administered mice were represented in Tables 4 and 5.
| Groups | Treatment | Biological parameters | |||
|---|---|---|---|---|---|
| ALP (U/L) | AST (U/L) | ALT (U/L) | Plasma Total Bilirubin (mg/dl) | ||
| I | Normal control | 130.46 ± 6.76 | 48.36 ± 6.74 | 39.472 ± 4.24 | 0.143 ± 0.06 |
| II | Induction control (25% CCl4, 1 ml/kg) | 640.22 ± 8.40* | 169.36 ± 8.80** | 264.87 ± 5.91* | 1.24* ± 0.12 |
| III | Control (Silymarin 100 mg/kg)+CCl4 | 281.45 ± 7.60λ | 50.665 ± 6.72 α | 78.022 ± 1.25 λ | 0.242α ± 0.07 |
| IV | Polygonum amplexicaule methanolic extract (200 mg/kg)+CCl4 | 295.76 ± 4.48 | 54.52 ± 6.41 | 125.26 ± 4.58 | 0.172 ± 0.06 |
| V | Polygonum amplexicaule methanolic extract (300 mg/kg)+CCl4 | 273.29 ± 5.80 | 57.55 ± 6.01 | 63.26 ± 2.96 | 0.153 ± 0.02αβ |
| VI | Polygonum amplexicaule aqueous extract (200 mg/kg)+CCl4 | 221.64 ± 2.56 | 82.402 ± 4.40 | 90.73 ± 5.31 | 0.194 ± 0.02α |
| VII | Polygonum amplexicaule aqueous extract (300 mg/kg)+CCl4 | 264.42 ± 8.22 | 62.28 ± 8.44 | 73.37 ± 6.10 | 0.153 ± 0.02 |
| VIII | Polygonum amplexicaule ethanolic extract (200 mg/kg)+CCl4 | 209.39 ± 10.1αβ | 71.446 ± 1.62 | 65.141 ± 2.2 | 0.273 ± 0.04λ |
| IX | Polygonum amplexicaule ethanolic extract (300 mg/kg )+CCl4 | 234.16 ± 7.81 | 57.34 ± 3.89 | 82.93 ± 7.41 | 0.163 ± 0.01 |
Values expressed as means ± S.D (n=6)
Values significantly varied comparable to normal control: *P<0.001, **P<0.01
Values significantly varied comparable to CCl4-administered control: αP<0.01, λP<0.05
Values significantly varied comparable to Drug control: βP<0.05
Table 4: Effect of Polygonum amplexicaule extracts on serum enzymes and plasma total bilirubin.
| Treatment | ALP (U/L) | AST (U/L) | ALT (U/L) | Plasma Total Bilirubin (mg/dl) |
|---|---|---|---|---|
| Control (Silymarin 100mg/kg)+CCl4 | 70.40% | 98% | 82.9 | 88% |
| P.A methanolic extract (200mg/kg)+CCl4 | 67.60% | 95% | 62% | 93.85% |
| P.A methanolic extract (300mg/kg)+CCl4 | 72% | 92% | 90% | 96% |
| P.A aqueous extract (200mg/kg)+CCl4 | 82.10% | 71.80% | 77.02% | 92% |
| P.A aqueous extract (300mg/kg)+CCl4 | 73.80% | 88.40% | 85% | 96% |
| P.A ethanolic extract (200mg/kg)+CCl4 | 84.50% | 81% | 89% | 85% |
| P.A ethanolic extract (300mg/kg )+CCl4 | 80% | 92% | 81% | 94.40% |
Table 5: Percent of protection of various biochemical parameters, as compared to induction control group (CCl4 group).
CCl4-administered mice exhibited a very highly significant increase (P<0.001) of ALT level (264 ± 5.91) as compared to normal control (39.472 ± 4.24). Elevation of serum ALT clearly indicated the toxic effect of CCl4 cause extensive damage to the liver because it is localized in cytoplasm and released after cellular damage [25].
The pretreatment of CCl4-administered mice with rhizome extracts of plant produced a very significant decrease (P<0.01) in case of ethanolic extracts (65.141 ± 2.2) at concentration of 200 mg/kg b.w and decrease (P<0.05) in case of aqueous extract 200 mg/kg (90.73 ± 5.31), aqueous extract 300 mg/kg (73.37 ± 6.10) and ethanolic extracts (82.93 ± 7.41) at concentration of 300 mg/kg b.w as compared to CCl4 administered control group. These extracts when compared with standard drug (78.02 ± 1.25) revealed that aqueous extract and ethanolic extracts are significantly varied (P<0.05) to drug. Several studies have been reported on hepatoprotective activity of medicinal plants. Among these, results of ALT reported by Pendit et al. [26] were quite similar to our present studies. ALT level of pretreated CCl4- administered rats with Adhatoda vasica was reported as 49.0 ± 5.9 as compared to CCl4-administered control group (Figures 2 and 3).
AST activity
AST presents two isozymes, one is present in mitochondria while the other one in cytoplasm [27]. Data are showing the significant rise in AST level in toxic control while highly significant decrease in groups treated with different concentrations of extracts.
It has been found highly significant rise (P<0.05) in serum AST level in CCl4–administered control group (169.362 ± 2.17) as compared to normal control taken as negative control (48.36 ± 7.79).
The pretreated mice with plant extracts have significantly lowered (P<0.01) AST level. Methanolic, ethanolic, aqueous extracts of plant having 300 mg/kg dose and methanolic extracts of 200 mg/kg showed same effect on lowering AST level up to 57.55 ± 6.01, 57.34 ± 3.89, 62.28 ± 8.44 and 54.52 ± 6.41 respectively as compared to CCl4-administered toxic control having AST value of 169.36 ± 8.8. Ethanolic and aqueous extracts were further showed significant variation (P<0.05) when compared with standard drug control having AST of 50.665 ± 6.7. Pendit et al. [26] have reported AST level in case of pretreated rat with plant extract but with dose of 200 mg/kg b.w which is close to our study.
ALP activity
ALP is present in hepatocytes plasma membrane and elevation in ALP level is related directly to the damage in liver cell membrane [28,29]. Elevated level of ALP in toxic condition and reduced level in case of treatment with drug and extracts of P. amplexicaule is shown.
Results indicated in Table 4 show significant increase of ALP in CCl4-administered control group as compared to normal non-toxic group. Normal value of ALP in mice were found to be 130.46 ± 6.76 which rose significantly (P<0.001) to respective value of 640.22 ± 8.40 after administration of toxic dose of CCl4 (1 ml/kg).
Pretreatment of CCl4 administered mice with different extracts of P. amplexicaule returned the values to normal indicates the preventive effects of plant. Among these extracts, ethanolic and aqueous extracts of 200 mg/kg returned the serum ALP level to 209.39 ± 10.1 and 221.64 ± 2.56 respectively which were significantly lower (P<0.01) then the value of toxic control and non-significantly (P>0.05) close to normal values. The values also significantly (P<0.05) varied when compared with drug control (281.45 ± 7.60) compared to the lowering level of ALP reported by Pendit et al. [26].
Plasma bilirubin level
Serum bilirubin level estimation is another one of the most important test used for hepatic diseases diagnosis which measures the binding, conjugation and excreting capacity of hepatocytes proportional to erythrocytes degradation rate [23]. The present data shown the bilirubin level in normal, diseased and mice pretreated with different extracts of plant sample.
Result showed in Table 4 indicated that in case of CCl4- administered group there is a highly significant rise (P<0.001) in the level of plasma bilirubin (1.19 ± 0.12) as compared to non-treated mice (0.246 ± 0.06). This increased level of serum bilirubin is due to excessive production of bilirubin because of breakdown of RBCs and the inability of animal to excrete bilirubin due to liver damage [29]. The increase of plasma bilirubin levels by CCl4 further confirmed that CCl4 is toxic for liver which agrees with Ahsan et al. [16]; Vogel [30]; Samudram et al. [31].
Among these treatments results significantly lowered (P<0.01) in case of ethanolic and aqueous extracts of 200 mg/kg having respective bilirubin levels of 0.273 ± 0.04 and 300 mg/ kg 0.194 ± 0.02 and methanolic extract of 300 mg/kg 0.153 ± 0.03 as compared to CCl4 toxic control. Further comparison of these extracts with standard drug revealed that among these methanolic extracts is significantly varied (P<0.05) which is closely related with the results as described by Krishna et al. [21].
Acknowledgment
The authors are thankful to the financial support from Pakistan Science Foundation (PSF) under project PSF/Res/P-UAAR/ BIOTECH.
References
- Sundari PN, Wilfred G, Ramakrishna B (1997) Does oxidative protein damage play a role in the pathogenesis of CCl4 induced liver injury in the rats? Biochem Biophys Acta 1342: 169-176.
- Bahcecioglu IH, Ustundag B, Ozercan I, Ercel E, Baydas G, et al. (1999) Protective effects of Ginkgo biloba extract on CCl4 induced liver damage. Hepatol Res 15: 215-224.
- Shenoy KA, Somayaji SM, Bairy K L (2001) Hepatoprotective effects of Ginkgo biloba in CCl4 induce hepatic injury in rats. Ind J Pharmacol 33: 260-266.
- Peng C, Chunying L, Wenqaing P, Yue Z, Wei D, et al. (2009) The protective role of per2 against CCl4 induced hepatotoxicity. Am J Pathol 174: 63-70.
- Hogade MG, Patil KS, Wadkar GH, Mathapati SS, Dhumal PB (2010) Hepatoprotective activity of Morusalba (Linn.) leaves extract against CCl4 induced hepatotoxicity in rats. African J Pharma Pharmacol 4: 731-734.
- Karbownik M, Lewinski A, Reiter RJ (2001) Anticarcinogenic action of melatonin which involves antioxidative processes: Comparison with other antioxidants. Int J Biochem Cell Biol 33: 735-743.
- Reilly PM, Bulkley GB (1990) Tissue injury by free radicals and other toxic oxygen metabolites. Brit J Surg 77: 1324-1325.
- Chattopadhyay RR (2003) Possible mechanism of hepatoprotective activity of Azadirachtaindica leaf extract: part II. J Ethnopharmacol 89: 217-219.
- Sabeen M, Ahmad SS (2009) Exploring the folk medicinal flora of Abbottabad City, Pakistan. Ethnobot Leaflets 13: 810-833.
- Hussain J, Khan AL, Rehman N, Khan ZF, Hussain ST, et al. (2009) Proximate and nutrient investigation of selected medicinal plant species in Pakistan. J Nutr 8: 620-624.
- Uniyal SK, Singh KN, Jamwal P, Lal B (2006) Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed 2: 1-14.
- Runyoro DKB, Ngassapa OD, Matte MIN, Joseph CC, Moshi MJ (2006) Medicinal plants used by Tanzanian traditional healers in the management of Candida infections. J Ethnopharmcol 106: 158-165.
- Parekh J, Karathia N, Chanda S (2006) Evaluation of antibacterial activity and phytochemical analysis of Bauhinia variegata L bark. Afr J Biomed Res 9: 53-56.
- Isobe T, Kanazawa K, Fujimura M, Noda Y (1981) Flavonoids of Polygonum sieboldi and Polygonum fuliforme. Bull Chem Soc Jpn 54: 32-39.
- Matin A, Khan MA, Ashraf M, Qureshi RA (2001) Traditional use of herbs, shrubs and trees of Shogran Valley, Mensehra, Pakistan. Pak J Biol Sci 4: 1101-1107.
- Ahsan R, Monir ul Islam KM, Musaddik A, Haq E (2009) Hepatoprotective activity of methanol extract of some plants against CCl4 induced hepatotoxicity in albino rats. Global J Pharmacol 3: 116-122.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Path 28: 56-63.
- Res GSCC (1972) Optimized standard colorimetric methods. J Clin Chem Biochem 10: 182.
- Jendrassik L, Grof P (1938) Vere infechte photometrische Methodenzur Besimmung des Bilirubins. Biochem 297: 81-89.
- Rawat AK, Mehrotra S, Tripathi SC, Shome U (1997) Hepatoprotective activity of Boerhaavia diffusa L. roots a popular Indian ethnomedicine. J Ethno Pharmcol 56: 61-66.
- Krishna KI, Mruthunjaya K, Patel JA (2010) Antioxidant and hepatoprotective potential of stem metanolic extracts of Juticia gendarussa Burn. Int J Pharm 6: 72-80.
- El-Shenaway NS, Al-Eiza RA, El-Salmy F, Salah O (2009) Profylectic effect of vitamin E against hepatotoxicity, nephrotoxicity and histopathology induced by diazone insecticide in mice. Curr Zool 55: 219-226.
- Singh B, Chandan BK, Prabarkar A, Taneja SC, Singh J, et al. (2005) Chemistry and hepatoprotective activity of an active fraction from Baleriaprionitis in experimental animals. Phytother Res 19: 391-404.
- El-Tawil OS, Rehman MSA (2001) The role of enzyme induction and inhibition on cypermethrin toxicity. Pharmacol Res 44: 33-40.
- Mohan KG, Pallavi E, Ramesh M, Vankatesh S (2007) Hepatoprotective activity of Ficuscarica leaf extract against CCl4 induced hepatotoxicity in rats. DARU 15: 162-166.
- Pendit S, Sur TK, Jana U, Debnath PK, Sen S, et al. (2004) Prevention of CCl4 hepatotoxicity in rats by Aghatoda vasica leaves. Ind J Pharmacol 36: 312-320.
- Sapakal VD, Ghadge RV, Adnaik RS, Naikwade NS, Magdum CS (2008) Comparative hepatoprotective activity of LIv-52 and Livomyn against CCl4 induced hepatic injury in rats. Inter J Green Pharm 2: 79-82.
- Dahiru D, Oboidoa O (2007) Evaluation of the antioxidant effects of ziziphus mauritiana Lam. Leaf extract against chronic ethanol induced hepatotoxicity in rat liver. Afr J Tradit Complement Alterna Med 5: 39-45.
- Ahmad A, Pillai KK, Najmi AK, Ahmad SJ, Pal SN, et al. (2002) Evaluation of hepatoprotective potential of jigraine poast treatment against thioacetamide induced hepatic damage. J Ethnopharmacol 79: 35-41.
- Vogel HG (2002) CCl4 induced liver fibrosis in rats. In: Vogel HG, Vogel WH (eds.) Drud delivery and evaluation, Pharmacological assays (2nd edn.), Berlin: Springer Verlag: p: 942.
- Samudram P, Rajeshwari H, Vasuki R, Geeta A, Sathiya MP (2008) Hepatoprotective activity of bi herbal ethanolic extract on CCl4 induced hepatic damage in rats. J Afr Biochem Res 2: 61-65.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences