PI*ZMwurzburg: A Novel Rare Genotype of Severe Alpha 1-Antitrypsin Deficiency
Claudio Tirelli*, Davide Piloni, Francesca Mariani, Stefania Ottaviani, Ilaria Ferrarotti and Angelo Guido Corsico
1Department of Internal Medicine and Therapeutics, Division of Respiratory Diseases, University of Pavia and IRCCS Policlinico San Matteo Foundation, 27100, Pavia, Italy
2Division of Respiratory Diseases, IRCCS Policlinico San Matteo Foundation, 27100, Pavia, Italy
3Center for Diagnosis of Inherited Alpha 1-antitrypsin Deficiency, Department of Internal Medicine and Therapeutics, University of Pavia, 27100, Pavia, Italy
- *Corresponding Author:
- Claudio Tirelli
Department of Internal Medicine and Therapeutics
Division of Respiratory Diseases
University of Pavia and IRCCS Policlinico San Matteo Foundation
Viale Camillo Golgi, Pavia, Lombardy, 27100, Italy
Tel: 00989121909095
E-mail: claudio.tirelli01@ateneopv.it
Received Date: June 23, 2020; Accepted Date: July 16, 2020; Published Date: July 23, 2020
Citation: Tirelli C, Piloni D, Mariani F, Ottaviani S, Ferrarotti I, et al. (2020) PI*ZMwurzburg: A Novel Rare Genotype of Severe Alpha 1-Antitrypsin Deficiency. J Rare Disord Diagn Ther Vol.6 No.4:4. doi: 10.36648/2380-7245.6.4.201
Abstract
Background and Context: Alpha 1-antitrypsin deficiency (AATD) is a rare autosomal codominant disease typically leading to early development of emphysema due to imbalance between neutrophilic proteases and Alpha 1-antitrypsin (AAT) antiprotease effect. AAT polymerogenic variants, as Z, or rare Mmalton and Mwurzburg, can also contribute to liver fibrosis.
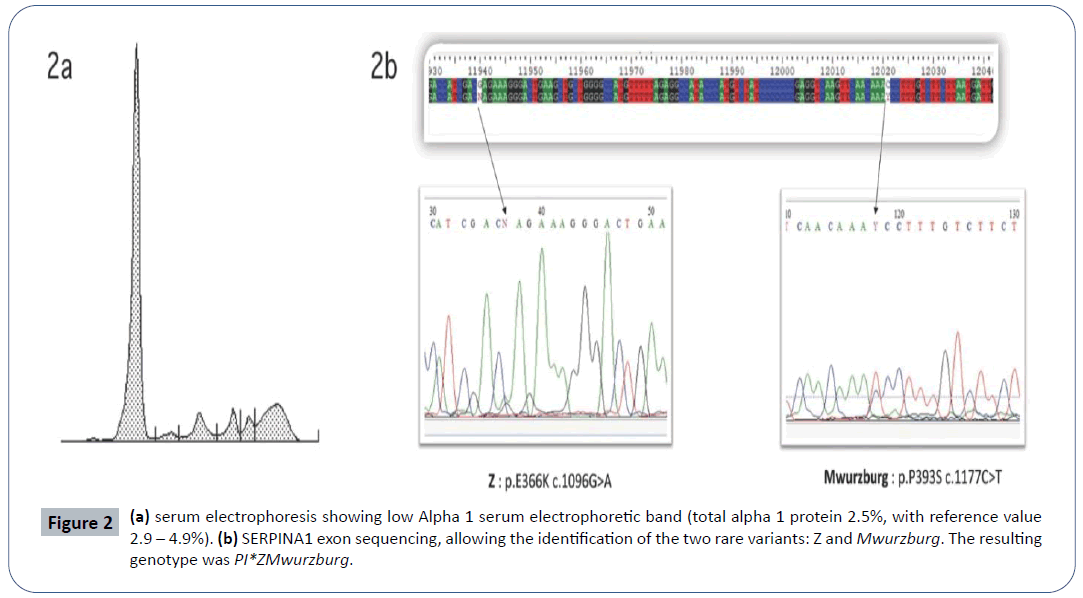
Case report: We describe a novel rare genotype of severe AATD identified in a 16-year-old asthmatic never-smoker male suffering for spontaneous left-apical pneumothorax, recurrent pneumonias and hepatic fibrosis. Suspicion of AATD was posed after a low alpha 1 serum electrophoretic band (total alpha 1 proteins 2.5%, reference value 2.9-4,9%) was detected. AAT blood concentration resulted 51.8 mg/dL (reference value 90-200 mg/dL).
Discussion: Genetic analysis demonstrated the presence in heterozygote fashion of two deficient variants: Z (p.E366K, c.1096G>A, rs28929474) and Mwurzburg (p.P393S, c.1177C>T, rs28931570). Therefore, genotype resulted PI*ZMwurzburg. Despite severe AATD, since the very young age of the patient, the possibility to avoid risk factors as smoke and alcohol intake, and the moderate pulmonary clinical involvement, a strict follow up without AAT augmentation therapy was applied.
Conclusion: When AATD is suspected, correct genotype should be always achieved through molecular analysis to personalize clinical-therapeutic management of this rare disorder.
Keywords
Alpha 1-antitrypsin deficiency (AATD); Rare Genotypes; Asthma; Liver Fibrosis; SERPINA1
Introduction
Alpha 1-antitrypsin (AAT) is a 52 kDa acute phase glycoprotein encoded by SERPINA1 gene at chromosome 14q32.1. It is synthesized by hepatocytes, and to a lesser extent, by lung and intestinal epithelial cells, neutrophils and macrophages. AAT acts as an anti-inflammatory protein mainly in the lung, as serine protease inhibitor against neutrophil elastase, which is released by neutrophils during inflammation causing proteolysis of lung connective tissue and cell damage [1]. Alpha 1-antitrypsin deficiency (AATD) is a rare autosomal codominant disease typically leading to early development of basal emphysema. Moreover, similarly to what happens in usual COPD, an important adaptive immune inflammation has been described as a prominent feature in AATD [2]. Other manifestations include child and adult liver disease, with fibrotic changes, due to misfolded AAT accumulation in hepatocytes, while rarely AATD has been associated with vasculitis and panniculitis [3]. The most common mutations in AATD are the severe Z and mild S variants (respectively associated with 10-15% and 55-60% AAT serum levels). Patients with PI*ZZ genotype have severe plasma AAT deficient levels, which means lower than 50 mg/dL or 11 μmol. Moreover, in the literature about 100 rare variants have also been described, both as deficient variants or null [4]. The resulting imbalance between neutrophilic proteases and the AAT-antiprotease effect gradually leads to the development of emphysema. However, AATD could also manifest with fibrotic liver disease, due to production of misfolded AAT which tends to polymerize with subsequent accumulation in the endoplasmic reticulum of hepatocytes. In addition to Z mutation, other rarer variants, as Mmalton and Mwurzburg, have been linked to the production of misfolded AAT [5]. The current standard of care for patients affected by AATD-associated emphysema is replacement therapy by weekly intravenous infusion of pooled human plasma purified AAT that reduces the progression of emphysema as assessed by CT-densitometry [6]. We reported a case of a young male suffering from severe AATD with the rare genotype PI*ZMwurzburg, manifested with asthma, recurrent respiratory infections, spontaneous PNX and mild hepatic fibrosis. To the best of our knowledge, this is the first reported case of PI*ZMwurzburg AATD, with both lung and liver involvement.
Case Report
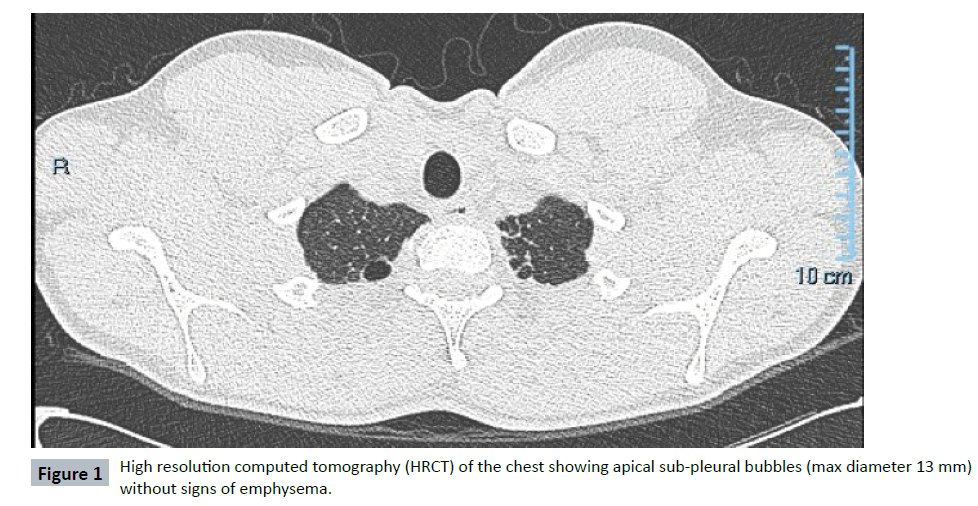
In 2013, a 16-year-old Caucasian never-smoker male was admitted to a local hospital for pneumonia and chest pain. In his past medical history, he suffered from recurrent bronchitis and pneumonias. He had also a history of allergic asthma (allergy for dog-epithelium, pellitory and dust mites) successfully treated with ICS/LABA (beclomethasone/formoterol). No family history of pulmonary disease was reported. At chest-X-ray a left-apical pneumothorax (PNX) was diagnosed and conservatively treated. Antibiotics were administered for pneumonia. To further investigate recurrent infections and spontaneous PNX, the patient was referred to the Respiratory Clinic of the IRCCS Policlinico San Matteo (Pavia-Italy). A chest high resolution computed tomography (HRCT) showed apical sub-pleural bubbles (max diameter 13 mm), some bilateral ground-glass alterations, without mediastinal adenomegaly (Figure 1). Spirometry was normal, without obstruction (FEV1/ FVC 87%) or DLCO impairment (DLCO 109%) and no desaturation on exercise-test (Bruce’s modified protocol). On routine blood tests, a low alpha 1 serum electrophoretic band (total alpha 1 proteins 2,5%, reference value 2,9-4,9%) was detected (Figure 2a). In the suspicion of AATD, blood samples were collected for biochemical and molecular analysis. AAT blood concentration resulted 51,8 mg/dL (reference value 90-200 mg/dL). Genetic analysis demonstrated the presence in heterozygote fashion of two deficient variants: i) Z (p.E366K c.1096G>A rs28929474) and ii) Mwurzburg (p.P393S c.1177C>T rs28931570) (Figure 2b). Therefore, the genotype resulted PI*ZMwurzburg. Due to possible hepatic involvement related to Z and Mwurzburg polymerogenic variants, abdomen ultrasounds and hepatic sonoelastographytest (Fibroscan®) were performed: no hepatomegaly was found, but hepatic elastance (7.7 KPa) was compatible with mild liver fibrosis, which was confirmed at hepatic biopsy. Due to the young age and the possibility to avoid all risk factors (smoke, alcohol, fatty diet) linked to a rapid progression of the hepatic and lung involvement, the patient was strictly followed up. Although in the presence of a severe AATD, the very young age of the patient, the absence of emphysema detected at HRCT and the lack of DLCO impairment at spirometry, suggested not to immediately start AAT augmentation therapy. A conservative approach and a strict follow up with repeated spirometry (every 6 months) were preferred. Possible progression of hepatic fibrosis has been also monitored with yearly hepatic sonoelastography-tests. Till now (7 years follow up), no liver fibrosis progression has been observed, no other PNX occurred and spirometry is stable. In case signs of lung function deterioration (at spirometry or HRCT), infusions of pooled human plasma purified AAT will be promptly started.
Discussion
We reported the first case of severe AATD with a rare genotype PI*ZMwurzburg, with both lung and liver involvement. In particular, detection of Mwurzburg variant is sporadic. A previous report on AATD screening in Tunisian COPD population found one patient with PI*MMwurzburg genotype, and mean AAT serum level of 0.91 g/L [7]. The AAT screening of a village in an Italian alpine valley detected 4 PI*MMwurzburg subjects [8]. The defective allele Mwurzburg is linked to intracellular AAT accumulation, with subsequent serum levels of approximately 20% of the normal.
The Mwurzburg variant, as well as the Z one, has polymerogenic properties, possibly leading to intracellular accumulation of misfolded alpha 1-antitrypsin in the endoplasmic reticulum of hepatocytes which subsequent hepatocellular damage and liver fibrosis [9,10]. Moreover, a recent paper demonstrated in vitro the increased intracellular accumulation of AAT variants when co- expressed with Z AAT, thus suggesting that the presence of two polymerogenic variants could likely explain the early intracellular co-polymerization in hepatocytes [11].
Conclusion
The suspect of AATD was based on serum electrophoresis because of low alpha 1 band, history of recurrent respiratory infections and asthma. The diagnosis was reached thanks to the combination of biochemical and genetic analysis which allowed the definition of the precise deficient genotype, which is mandatory to better address the clinical follow up. As a matter of fact, it proved very useful to rapidly investigate on possible liver fibrosis. Moreover, as suggested by recent statements, the precise genotyping at reference centres to detect rare variants other than most common Z and S alleles might prove very useful to reach the correct diagnosis. This could help to personalize clinical-therapeutic management of rare disorders as AATD.
References
- Silva D, Oliveira MJ, Guimarães M, Lima R, Gomes S, et al. (2016) Alpha-1-antitrypsin (SERPINA1) mutation spectrum: Three novel variants and haplotype characterization of rare deficiency alleles identified in Portugal. Resp Med 116: 8-18.
- Baraldo S, Turato G, Lunardi F, Bazzan E, Schiavon M, et al. (2015) Immune Activation in α1-Antitrypsin-Deficiency Emphysema. Beyond the Protease–Antiprotease Paradigm. Am J Respir Crit Care Med 191: 402-409.
- Miravitlles M, Dirksen A, Ferrarotti I, Koblizek V, Lange P, et al. (2017) European Respiratory Society statement: Diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Resp J 50: 1700610.
- Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, et al. (2016) α1-Antitrypsin deficiency. Nat Rev Dis Primers 2: 16051.
- Gooptu B, Dickens JA, Lomas DA (2014) The molecular and cellular pathology of α₁-antitrypsin deficiency. Trends Mol Med 20: 116-127.
- Ferrarotti I, Ottaviani S, De Silvestri A, Corsico AG (2018) Update on α1-antitrypsin deficiency. Breathe 14: e17-e24.
- Denden S, Zorzetto M, Amri F, Knani J, Ottaviani S, et al. (2009) Screening for Alpha 1 antitrypsin deficiency in Tunisian subjects with obstructive lung disease: a feasibility report. Orph J Rare Dis 4: 12.
- Corda L, Medicina D, La Piana GE, Bertella E, Moretti G, et al. (2011) Population genetic screening for alpha1-antitrypsin deficiency in a high-prevalence area. Respiration 82: 418-425.
- Fra AM, Gooptu B, Ferrarotti I, Miranda E, Scabini R, et al. (2012) Three new alpha1-antitrypsin deficiency variants help to define a C-terminal region regulating conformational stability and polymerization. PLoS One 7: e38405.
- Poller W, Merklein F, Schneider-Rasp S, Haack A, Fechner H, et al. (1999) Molecular characterization of the defective α1-antitrypsin alleles PI Mwürzburg (Pro369Ser), Mheerlen (Pro369Leu), and Q0lisbon (Thr68lle). Eur J Hum Gen 7: 321-331.
- Laffranchi M, Berardelli R, Ronzoni R, Lomas DA, Fra A, et al. (2018) Heteropolymerization of α-1-antitrypsin mutants in cell models mimicking heterozygosity. Hum Mol Genet 27: 1785-1793.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences