Hypoparathyroidism – Review of the Literature 2018
Bridget P Sinnott
DOI10.21767/2380-7245.100180
Bridget P. Sinnott*
Division of Diabetes, Endocrinology and Metabolism, Medical College of Georgia, 1447 Harper St, HB-5025, Augusta, GA 30912, USA
- *Corresponding Author:
- Bridget P. Sinnott
Sinnott, Division of Diabetes
Endocrinology and Metabolism
Medical College of Georgia
1447 Harper St, HB-5025, Augusta, GA 30912, USA
Tel: +706-721-2131
Fax: +706-721-6892
E-mail: bsinnott@augusta.edu
Received Date: June 19, 2018; Accepted Date: June 29, 2018; Published Date: July 07, 2018
Citation: Sinnott BP (2018) Hypoparathyroidism – Review of the Literature 2018. J Rare Disord Diagn Ther. 4:12. doi: 10.21767/2380-7245.100180
Abstract
Hypoparathyroidism is a rare endocrine disorder characterized by low calcium and high phosphate levels, in the setting of a low or inappropriately normal PTH level. Given its rarity, it has been classified as an orphan disease in the United States and by the European Commission. The first international conference on the management of hypoparathyroidism was convened in Florence Italy 2015 and resulted in the publication of detailed guidelines on the epidemiology, presentation, clinical features and management of hypoparathyroidism. Hypoparathyroidism may be associated with a spectrum of clinical manifestations, ranging from asymptomatic in the setting of mild hypocalcemia, to life threatening cardiac arrythmias, seizures or laryngospasm, in the setting of severe acute hypocalcemia. The most common cause is postsurgical hypoparathyroidism following anterior neck surgery, followed by autoimmune disorders and rarely genetic disorders. Establishing a diagnosis of hypoparathyroidism is important as it is associated with the development of kidney stones, renal insufficiency, cataracts, basal ganglia calcifications, as well as a reduced health-related quality of life.
Most patients with chronic hypoparathyroidism require lifelong high dose calcium and activated vitamin D supplements. The goals of treatment are 3 fold, namely, relieve symptoms of hypocalcemia, raise calcium concentration to the low-normal range and avoid hypercalciuria. For those individuals who cannot maintain a stable serum and urine calcium with conventional calcium and activated Vitamin D regimens, the addition of recombinant PTH (1-84) is currently an option. The approval of recombinant PTH (1-84) represents a significant step forward in the management of this disorder.
https://blogum.blogaaja.fi/
https://blogum-1.jimdosite.com/
https://blogummm.edublogs.org/
https://blogummm.websites.co.in/
https://blogum18.wordpress.com/
https://benim-blogum.jigsy.com/
https://fuiegs-symbeaurds-build.yolasite.com/
https://blogum-03.webselfsite.net/
https://blogummm.mystrikingly.com/
https://blogum.splashthat.com/
https://blogum3.webnode.com.tr/
https://blogum.odoo.com/
https://blogum.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/enrollments
https://blogum.estranky.cz/
https://653ba4fbb538c.site123.me/
https://blogum12m.blogspot.com/
https://blogum.hashnode.dev/
https://whiteseoturkey1.wixsite.com/blogum
https://sites.google.com/view/blogummm/
https://codepen.io/blogum
https://blogumm.livejournal.com/
https://wakelet.com/@blogum82816
https://www.homify.com/users/9538383/blogum
https://lessons.drawspace.com/profile/323613/blogum
https://my.desktopnexus.com/blogum/
https://writeupcafe.com/profile/BLOGUM/
https://www.pearltrees.com/blogum
https://www.easyfie.com/blogum
https://pharmahub.org/members/27615/profile
https://www.zupyak.com/u/blogum/posts
https://www.metroflog.co/blogum
https://www.fuzia.com/fz/blogum-blogum
https://tr.pinterest.com/blogum12/
https://my.getjealous.com/blogum
https://micro.blog/blogum
https://www.tumblr.com/blogummm
https://hub.docker.com/u/blogum
https://fire.blogfree.net/?act=Profile&MID=1342323
https://blogum.pixnet.net/blog
https://www.threadless.com/@blogum/activity
https://blogum.neocities.org/
https://blogum12.amebaownd.com/
https://teletype.in/@blogum
https://ubl.xml.org/users/blogum
https://educatorpages.com/site/blogum/
https://blogum.onlc.fr/
Keywords
Chronic hypoparathyroidism; Hypocalcemia; Calcitriol; Recombinant PTH
Introduction
Hypoparathyroidism is a rare endocrine disorder characterized by low calcium and high phosphate levels, in the setting of a simultaneously low or inappropriately normal PTH level. Hypoparathyroidism has been classified as an orphan disease in the United States and by the European Commission. Many clinicians have limited experience or expertise in treating this specialized disorder. Fortunately, within the last 3 years, 3 sets of guidelines have been published by experts in the field of hypoparathyroidism, addressing the epidemiology, clinical presentation and features, as well as management of hypoparathyroidism [1-3]. Until recently the only treatment options available for chronic hypoparathyroidism included calcium supplements, activated vitamin D and thiazide diuretics. In the last few years recombinant human (rh) PTH (1-84) has been approved for the treatment of hypoparathyroidism and is a useful therapeutic option for patients with suboptimal calcium levels despite high dose calcium and activated vitamin D. In this paper, we will review hypoparathyroid pathophysiology, epidemiology, etiologies, diagnosis, clinical features, conventional treatment options, as well as recent advancement in treatment options available to date.
Parathyroid Hormone Pathophysiology
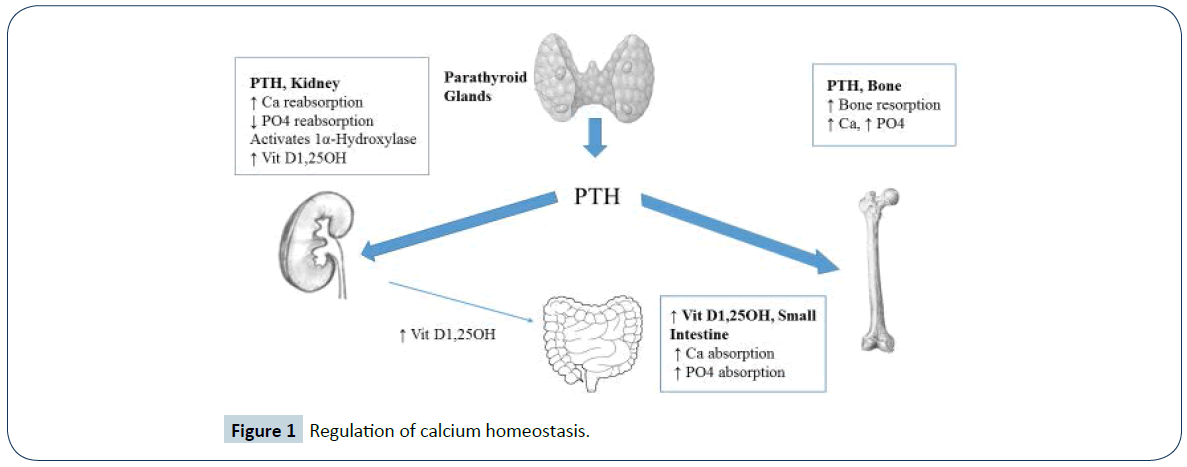
PTH, along with vitamin D are the major hormonal regulators of calcium homeostasis in the human body (Figure 1). PTH achieves this by direct effects on the kidney & bone and indirect effects on the gastrointestinal tract via production of activated Vit D1,25OH in the kidney. PTH secretion is regulated by the calcium sensing receptor (CaSR) which is located on parathyroid cells and on renal tubules. When the CaSR is stimulated by a low calcium level, PTH is secreted and increases calcium availability in the body.
PTH mobilises calcium from the skeleton and facilitates Vit D1,25OH production by the kidney, which leads to increased intestinal calcium absorption. Additionally, at the level of the kidney, PTH stimulates renal tubular calcium reabsorption and phosphate excretion by the kidney. When PTH secretion is inadequate or absent, hypocalcemia and hyperphosphatemia develop and represent the hallmark electrolyte abnormalities of this disorder. Functional hypoparathyroidism, related to severe hypomagnesemia, results in impaired PTH secretion and action [4]. This is a reversible cause of hypocalcemia associated with a low PTH level. Pseudohypoparathyroidism is a rare inherited disorder of PTH resistance at the level of the kidney that can lead to hypocalcemia. It is distinguished from chronic hypoparathyroidism by a high PTH level [5].
Epidemiology
Hypoparathyroidism is rare, with an estimated prevalence in the USA of 37 per 100,000 person-years and 22 per 100,000 person-years in Denmark [6]. Overall, an estimated 60 to 115,000 individuals are diagnosed with chronic hypoparathyroidism in the United States [6,7]. Most of cases result from anterior neck surgery, constituting up to 75% of all cases of hypoparathyroidism [6,8]. The remaining 25% of cases are non-surgical or more rarely, related to genetic disorders (Table 1).
| SYNDROMIC | OMIM [38] | Inheritance | Gene / Protein |
Chromosomal Abnormality | Clinical Features [38] |
|---|---|---|---|---|---|
| Polyglandular autoimmune syndrome type 1 | 240300 | AR | AIRE gene | 21q22.3 | 2 out of 3 of the following: Hypoparathyroidism, Addisons, and / or chronic mucocutaneous candidiasis. |
| DiGeorge syndrome | 188400 | AD | TBX1, NEBL | 22q1.2/tbx1 | Hypocalcemia, cardiac malformations of outflow tract, infections, short stature, and learning difficulties. |
| CHARGE syndrome | 214800 | AD | CHD7, SEMA3E | 8q12.1-q12.2, 7q21.11 | Hypocalcemia, coloboma, heart anomalies, choanal atresia, mental and somatic developmental delay and deafness. |
| Kenny-Caffey TYPE 1, 2 | 244460 127000 |
AD, AR | TBCE, FAM111A | 1q42.3, 11q12.1 | Hypocalcemia, craniofacial anomalies, cortical thickening of long bones, short stature, visual abnormalities, and psychomotor retardation. |
| Hereditary deafness & renal dysplasia syndrome (HDR) | 146255 | AD | GATA3 | 10p14 | Hypocalcemia, sensorineural deafness, and renal disease. |
| Autosomal Dominant hypocalcemia 1, with Bartter syndrome type 5 | 601198 | AD | CaSR | 3q21.1 | Hypocalcemia, nephrocalcinosis, kidney stones, and basal ganglia calcification. |
| NON-SYNDROMIC | OMIM [38] | Inheritance | Gene / Protein |
Chromosomal Abnormality | Clinical Features [38] |
| Isolated Hypoparathyroidism | 146200 146200 307700 |
AD AR X-linked |
PTH GCM2 SOX3 |
11p15, 6p24.2 Xq26-27 |
Clinical manifestation of hypoparathyroidism |
| ADH1 | 601198 | AD | CaSR | 3q21.1 | Hypocalcemia, nephrocalcinosis, kidney stones, and basal ganglia calcification. |
| ADH2 | 615361 | AD | GNA11 | 19p13 | Clinical manifestation of hypoparathyroidism |
OMIM = Online Mendelian Inheritance of Man; AR = Autosomal Recessive; AD = Autosomal Dominant; ADH = Autosomal Dominant Hypoparathyroidism; AIRE = Autoimmune regulator 1; TBX1 = T-box 1; NEBL = nebulette; CHD7 = chromodomain helicase DNA-binding protein 7; SEMA3E = semaphoring 3E; TBCE = tubulin folding cofactor E; FAM111A = family with sequence similarity 111 member A; GATA3 = GATA-binding protein 3; CaSR = calcium-sensing receptor; GCM2 = glial cells missing homolog 2; SOX3 = SRY-related HMG box; GNA11 = G protein subunit alpha 11.
Table 1: Most common syndromic and non-syndromic causes of hypoparathyroidism.
Etiologies
Postsurgical hypoparathyroidism, secondary to anterior neck surgery, is the most common etiology of hypoparathyroidism and is considered permanent if present beyond 6 months post-op. Transient hypoparathyroidism is common post-op due to parathyroid gland “stunning”, with only 2-3% of patients developing permanent hypoparathyroidism, following anterior neck surgery [9]. The second most common etiology is autoimmune hypoparathyroidism, related to autoimmune polyglandular syndrome type 1 or isolated hypoparathyroidism due to activating antibodies to the CaSR. Severe hypomagnesemia is an uncommon but reversible cause of hypoparathyroidism [5]. There are reports of magnesium malabsorption and reversible hypoparathyroidism in healthy patients with chronic proton pump inhibitor use [10]. Genetic forms of hypoparathyroidism can be divided into syndromic and non-syndrome (Table 1). These forms of hypoparathyroidism are associated with abnormal parathyroid function or action causing isolated hypoparathyroidism or hypoparathyroidism with congenital multisystem or metabolic abnormalities [5,6]. The European guidelines on hypoparathyroidism recommend considering genetic testing and / or family screening in patients with hypoparathyroidism without a clear etiology [2]. Other rarer causes of hypoparathyroidism include infiltrative disorders such as hemochromatosis, metastases or granulomatous diseases, radiation induced destruction of parathyroid glands, hungry bone syndrome post parathyroidectomy and HIV infection [5,6].
Clinical Presentation
The presentation of hypocalcemia depends on the rate of development, chronicity, as well as the severity of the calcium drop over time. Acute hypocalcemia is most commonly seen following anterior neck surgery. It usually manifests with neuromuscular irritability with patient complaints of perioral numbness, paresthesias and twitching in their extremities. Classic exam findings include Trousseau’s and Chvostek’s signs. Acute life-threatening hypocalcemia can lead to the development of arrythmias, laryngospasm and seizures. More commonly, the clinical presentation can be subtle and nonspecific, related to chronic hypocalcemia and hyperphosphatemia. Occasionally hypocalcemia may be an incidental finding on a biochemical screening test. Chronic hypocalcemia and hyperphosphatemia, with a resultant elevated calcium-phosphate product can lead to soft tissue calcifications in the basal ganglia or nephrocalcinosis and kidney stones, especially in patients on high dose supplemental calcium and activated vitamin D [8,11]. Many hypoparathyroid patients report cognitive issues such as fatigue, brain fog and easy fatigability. When hypoparathyroid patients were compared to normal controls, patients with hypoparathyroidism had a higher incidence of anxiety, depression and lower quality of life scores [8,12,13].
Complications related to high doses of calcium and active vitamin D necessary to maintain a normal calcium level are not uncommon. Mitchell et al. performed a retrospective review of 120 hypoparathyroid patients and reported a high prevalence of hypercalciuria (38%), nephrocalcinosis (31%), reduction in GFR <60ml/min/1.73 m2 (41%) and basal ganglia calcification (52%) [14]. Other reported complications include psychiatric disorders, seizures, cardiovascular disease, cataracts and infections [15]. Despite the many morbidities associated with hypoparathyroidism and its treatment, there is no increased overall mortality [6].
Physical Exam Findings
Physical exam findings of acute hypocalcemia include Trousseau’s and Chvostek’s signs. A neck evaluation is mandatory to evaluate for a prior anterior neck surgery scar. It is important to look for clues for autoimmune disorders such as vitiligo, mucosal candidiasis or nail bed fungal infections [1]. Signs of chronic hypocalcemia with subsequent ectopic calcification would include cataracts, costovertebral angle tenderness related to kidney stones or movement disorders related to basal ganglia calcification. Congenital anomalies, hearing loss, failure to thrive or learning difficulties would suggest the possibility of a genetic syndrome [6].
Laboratory Findings
The biochemical hallmarks of hypoparathyroidism include hypocalcemia, hyperphosphatemia, hypercalciuria and a low or inappropriately normal PTH level, in the absence of hypomagnesemia. The most current guidelines recommend checking an albumin adjusted calcium level and PTH, on at least 2 occasions separated by at least 2 weeks [1]. Ideally an ionized or free calcium should be the most accurate measure of calcium however obtaining an accurate measurement is difficult and therefore not recommended for diagnosis in the most recent hypoparathyroid guidelines [1]. Importantly, PTH can be accurately measured by second and third generation assays [1]. It is important to get a magnesium level to evaluate for functional hypoparathyroidism. To restore normocalcemia in patients with hypomagnesemia, it is essential to first treat the hypomagnesemia. Urine calcium levels can be variable and depend on calcium supplement intake. Urine calcium excretion is typically increased in the setting of hypoparathyroidism, related to loss of the stimulatory effect of PTH on renal tubular calcium reabsorption [16]. Loss of the inhibitory effect of PTH on renal tubular phosphate reabsorption results in a reduced renal phosphate excretion and hyperphosphatemia. Activated Vit D1,25OH and bone turnover markers are usually within the low normal range [16]. After a diagnosis of hypoparathyroidism has been made, it is recommended that the following tests be obtained: phosphate, magnesium, Vit D 25OH, Vit D1,25OH, BUN/creatinine and 24-hour urine calcium and creatinine [1,2].
Imaging
The guidelines recommend checking a bone density by dual energy x-ray absorptiometry, skull x-ray for basal ganglia calcification and abdominal imaging for nephrocalcinosis or kidney stones [1]. Renal imaging is recommended every 5 years if asymptomatic and more frequently with nephrocalcinosis and stones [1] or an unexplained rise in creatinine [2].
Medical Management of Hypoparathyroidism
Management depends upon whether the presentation is acute or chronic and the severity of the hypocalcemia.
Conventional therapy
Acute hypocalcemia: Hypocalcemia can present acutely in the post-op anterior neck surgery setting, during acute illness, stress, in the luteal phase of the menstrual cycle or in the setting of medication non-compliance [8,11,17]. Presentations range from mild paresthesias, carpo-pedal spasms to more life-threatening complications such as seizures, arrythmias or laryngospasm. In the acute setting, namely patients with symptomatic hypocalcemia, acute decrease in serum calcium <7.5 mg/dL or patients with prolonged QT interval on EKG, hypocalcemia is typically managed with a bolus of 1-2 ampules (90-180 mg elemental calcium) of 10% calcium gluconate followed by IV calcium gluconate drip of 0.5-1.5 mg/kg/hour over 8-10 hours [1,17]. Oral calcium and activated vitamin D should be initiated as soon as the patient is able to tolerate, to facilitate weaning of IV calcium.
Chronic hypocalcemia: The goals of optimal treatment of hypoparathyroidism include maintaining a serum calcium within the low-normal range, serum phosphate and magnesium levels within the normal range, a calcium-phosphate product below 55 mg2/dL2, while avoiding hypercalciuria [17]. Standard treatment includes calcium supplements and activated vitamin D, namely calcitriol or alfacalcidol. Vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol) supplements, magnesium and thiazide diuretics are used as needed. Recombinant PTH is not recommended as 1st line treatment for hypoparathyroidism [1,2].
Dietary calcium intake should be encouraged based on the guidelines for the general population [18]. Calcium supplements are most commonly available as calcium carbonate, which contains 40% elemental calcium. In some circumstances, calcium citrate which contains 20% elemental calcium, is preferred. In contrast to calcium carbonate, calcium citrate does not require an acidic environment for absorption and is the optimal calcium replacement in the setting of achlorhydria or proton pump inhibitor use [19]. The absorptive capacity of the small intestine is saturated by intake of a dose of about 500 mg elemental calcium in one ingestion [20], therefore calcium supplements need to be split up into multiple daily doses, typically 2-4 times daily. Typical replacement doses in hypoparathyroidism range between 1-9 gms of elemental calcium daily [21], with most patients requiring 1-2 gm of elemental calcium daily. The target calcium level is the low-normal range and no more than 0.5 mg/dL below the normal range [1]. It is necessary to consider additional calcium supplementation, and even calcitriol, prophylactically with illness, stress, exercise or menses. There are a number of activated vitamin D analogues available. In the USA, calcitriol is available; in Europe alfacalcidol (1α-hydroxyvitamin D) and dihydrotachysterol are available. The dose of calcitriol required is highly variable and ranges between 0.25 and 2 mcg daily [1], which is equal to a daily dose of 0.5-4mcg of alfacalcidol [22]. Vitamin D2 (ergocalciferol) in high doses is another treatment option for chronic hypoparathyroidism and may be particularly useful in patients with recurrent hypocalcemia on calcium and calcitriol alone but can be associated with vitamin D toxicity [23]. Generally, vitamin D2 or vitamin D3 is administered to maintain Vit D25OH levels in the normal range for many tissues that generate their own Vit D1,25OH, which may have non-skeletal beneficial effects. The Institute of Medicine recommends a daily dose of Vitamin D2/D3 400-800 IU [18]. A serum concentration of Vit D25OH above 80nmol/L (30 ng/ml) is generally considered adequate [24]. During calcium and activated vitamin D dose adjustments, it is important to monitor the serum calcium, phosphate, magnesium and creatinine closely, up to weekly or more frequently, depending on the clinical scenario and until a stable calcium level is achieved [17]. Additionally, it is important to assess for symptoms of hypocalcemia and hypercalcemia, in order to assure that the treatment regimen provides relief of symptoms, without causing unnecessary side effects. Once patients are on a stable dosing regimen, every 3-6 months measurement of calcium may be adequate. It is necessary to check 24-hour urine calcium levels annually to evaluate for hypercalciuria and the need to reduce calcium or activated Vitamin D supplements [8,11,17]. If hypercalciuria persists, then a thiazide diuretic accompanied by a low salt diet is recommended and has been shown to be effective [25]. Thiazides stimulate distal renal tubular calcium reabsorption and may exert a calcium sparing effect, allowing for lower doses of calcium supplements. It is important to monitor serum potassium and magnesium given the renal loss of these electrolytes with thiazide use [11]. It is important to maintain a normal magnesium level. Risk factors for hypomagnesemia include use of diuretics and proton pump inhibitors [10]. Magnesium replacement with sustained release preparations minimizes renal excretion of magnesium and is associated with less gastrointestinal side effects. Typical requirements are 240- 1000 mg elemental magnesium in divided doses in patients with normal renal function [2]. Hyperphosphatemia is a feature of hypoparathyroidism and it is important to maintain the calcium-phosphate product below 55 mg2/dL2, to reduce the risk for extra-skeletal calcification. It may be necessary to titrate down calcitriol to reduce intestinal phosphate absorption and increase supplemental calcium intake, to act as an intestinal phosphate binder. Additionally, a low phosphate diet may be beneficial [8]. Conventional therapy with high dose calcium and activated vitamin D can be associated with many complications and doesn’t address the underlying deficiency in PTH. Recently, rhPTH therapy was approved and offers a more physiologic approach to the treatment of hypoparathyroidism.
Hormone Replacment Therapy
rhPTH (1-34), also known as Teriparatide®
rhPTH (1-34) is a truncated synthetic molecule of PTH, which was the first PTH replacement therapy available and is approved for the treatment of osteoporosis, but not chronic hypoparathyroidism. It was the first PTH replacement therapy studied in chronic hypoparathyroidism. Replacement therapy with twice daily rhPTH (1-34) has been studied in small cohorts of children and adults with chronic hypoparathyroidism, with promising results [26,27]. Use of PTH (1-34) administered continuously by a pump demonstrated a 59% significant reduction in urine calcium excretion, compared to twice daily injections rhPTH (1-34) [28]. Interestingly, a smaller daily dose of rh PTH (1-34) was required with pump delivery vs twice daily dosing regimens. Continuous delivery of PTH 1-34 via a pump, has provided the closest approach to date to physiological replacement therapy for hypoparathyroidism, with normalisation of the serum levels of calcium, phosphate and PTH levels.
rhPTH (1-84), also known as Natpara®
rhPTH (1-84) is the full length synthetic molecular form of PTH. Once daily rhPTH (1-84) was FDA approved 1/2015 for the management of chronic hypoparathyroidism, of any etiology, except Autosomal Dominant Hypocalcemia. It is indicated for patients with poorly controlled calcium levels on standard calcium and activated vitamin D therapy. The drug is available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). The FDA approved rhPTH (1-84) with a “black box” warning regarding the development of osteosarcoma in rats with PTH use but did not limit the duration of its use in humans with hypoparathyroidism. The European Commission granted Conditional Marketing Authorisation for rhPTH (1-84) 4/2017. Similar to the FDA, it is indicated as adjuvant treatment for adult patients with chronic hypoparathyroidism who cannot be adequately controlled with standard treatment alone. Approval of rhPTH (1-84) was based on the outcome of the pivotal phase III REPLACE trial [29]. REPLACE was a multinational, randomized, double blind, placebo controlled, phase III study in which 134 patients, aged 19-74 years, with chronic hypoparathyroidism were randomized in a ratio 2:1 to receive rhPTH (1-84) once daily or placebo for 24 weeks. The primary endpoint was a 50% or greater reduction from baseline in the daily dose of oral calcium and active vitamin D, while maintaining a stable albumin-corrected serum calcium concentration greater than or equal to baseline concentration. At the end of the study, 53% patients on rhPTH (1-84) achieved the primary endpoint compared with 2% of patients in the placebo group (p<0.0001). Additionally, 43% patients on rhPTH (1-84) were able to completely stop all active vitamin D versus 5% in the placebo group and reduce their calcium dose to 500 mg/daily or less, while maintaining normocalcemia (p<0.001). Replacement treatment with rhPTH (1-84) is an attractive option for patients with hypoparathyroidism who are unable to maintain stable and safe serum and urinary calcium levels on high dose calcium and activated vitamin D. rhPTH (1- 84) is a once daily subcutaneous injection which should be injected into the thigh. The starting dose is 50 mcg once daily and can be increased in 25 mcg increments to a max dose of 100 mcg daily. The serum calcium is monitored every 3-7 days after initiation and similarly when the dose is changed. Upon initiation of rhPTH (1-84), the dose of active vitamin D is reduced by 50%. The goals of treatment are 3-fold – to reduce supplemental calcium to 500 mg daily; minimize or eliminate active vitamin D treatment and to maintain a serum calcium in the lower range of normal [17]. Given the short half-life of rhPTH (1-84), clinicians need to be mindful that sudden discontinuation of rhPTH (1- 84) can lead to acute hypocalcemia and its life-threatening complications. The first international conference on the management of hypoparathyroidism recommend considering use of rhPTH (1-84) in the following individuals [1] – patients with inadequately controlled serum calcium levels; patients requiring >2.5 gm calcium or >1.5 mcg calcitriol daily; patients with renal complications of hypercalciuria or reduced eGFR < 60; patients with hyperphosphatemia and/or calcium-phosphate product that exceeds 55 mg2/dL2; patients with gastrointestinal disorders associated with malabsorption and those with a reduced quality of life.
Both the European guidelines [2] and guidelines from first international conference on hypoparathyroidism [1] recommend against the routine use of replacement rhPTH and recommend reserving it for select cases poorly controlled on conventional therapy. Moreover, long-term beneficial effects on outcomes such as hypercalciuria, renal complications and quality of life measures, have so far not been demonstrated in randomized control trials [26,29,30]. Since therapy with rhPTH (1-84) is a long-term management option, more long-term efficacy and safety data are needed in humans. There is currently safety and efficacy data through 6 years of rhPTH (1-84) use [31] and 7 years US post-marketing surveillance data on adults treated with rhPTH (1-34) for osteoporosis [32].
Parathyroid Gland Transplantation
The possibility of parathyroid gland allotransplants has emerged as a novel approach to manage and potentially cure chronic hypoparathyroidism. There are a number of case reports of successful parathyroid allotransplants in the literature. Parathyroid allotransplants have been performed successfully in therapy refractory postsurgical hypoparathyroid patients [33-35]. There has been several successful cases of simultaneous kidney and parathyroid allotransplants - one in a patient with postsurgical hypoparathyroidism related to ESRD [36] and one in a patient with congenital hypoparathyroidism complicated by nephrolithiasis and subsequent renal failure [37]. Successful parathyroid allotransplantation for hypoparathyroidism represents a novel strategy that could provide the definitive treatment for patients with difficult to control hypoparathyroidism. This remains an area for further research and investigation.
Conclusion
This article summarises our current state of knowledge on hypoparathyroidism and the advances in the diagnosis and management of this rare disorder. Until recently, hypoparathyroidism remained one of the last endocrine disorders without available hormone replacement therapy. The approval of rhPTH (1-84) represents an important step forward in the management of hypoparathyroidism, in patients poorly controlled with conventional therapy. It remains to be seen whether PTH replacement will reduce renal complication associated with the disease and its treatment, improve bone quality, reduce soft tissue calcification and improve quality of life measures. Other potential options on the horizon include parathyroid gland allotransplants.
References
- Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, et al. (2016) Management of Hypoparathyroidism: Summary Statement and Guidelines. J Clin Endocrinol Metab 101: 2273-2283.
- Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, et al. (2015) European Society of Endocrinology clinical guidelines: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol 173: G1-G20.
- Stack BC Jr, Bimston DN, Bodenner DR, Brett EM, Dralle H, et al. (2015) American Association of Clinical Endocrinologists and American College of Endocrinology Disease state clinical review: postoperative hypoparathyroidism – definitions and management. Endocr Pract 21: 674-85.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, et al. (1984) The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 310: 1221-1225.
- Thakker RV. Hypocalcemia: pathogenesis, differential diagnosis and management. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. 5th edition; American Society of Bone and Mineral Research. 42: 271-274.
- Clarke BL, Brown EM, Collins MT, Juppner H, Lakatos P, et al. (2016) Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 101: 2284-99.
- Powers J, Joy K, Ruscio A, H Lagast (2013) Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res 28: 2570-2576.
- Bilezikian JP, Khan A, Potts Jr, Brandi ML, Clarke BL, et al. (2011) Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment and challenges for future research. J Bone Miner Res 26: 2317-2337.
- Rafferty MA, Goldstein DP, Rotstein L, Asa SL, Panzarella T, et al. (2007) Completion thyroidectomy versus total thyroidectomy: is there a difference in complication rates? An analysis of 350 patients. J Am Coll Surg 205: 602-607.
- Epstein M, McGrath S, S Law (2006) Proton pump inhibitors and hypomagnesemia hypoparathyroidism. N Engl J Med 355: 1834-6.
- Shoback D (2008) Clinical practice. Hypoparathyroidism. N Engl J Med 359: 391-403.
- Arlt W, Fremerey C, Callies F, Reincke M, Schneider P, et al. (2002) Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol 146: 215-22.
- Cusano NE, Rubin MR, Irani D, Sliney J Jr, JP Bilezikian (2013) Use of parathyroid hormone in hypoparathyroidism. J Endocrinol Invest 36: 1121-1127.
- Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, et al. (2012) Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 97: 4507-4514.
- Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L (2014) Postsurgical hypoparathyroidism - risk of fractures, psychiatric diseases, cancer, cataracts, and infections. J Bone Min Res 29: 2504-2510.
- Hannan FM, Thakker RV (2013) Investigating hypocalcemia. BMJ 346: f2213.
- Bilezikian JP, Brandi ML, Cusano NE, Mannstadt M, Rejnmark L, at al. (2016) Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab 101: 2313-2324.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicans need to know. J Clin Endocrinol Metab 96: 53-58.
- Milman S, Epstein EJ (2011) Proton pump inhibitor-induced hypocalcemic seizure in a patient with hypoparathyroidism. Endocr Pract 17: 104-107.
- Harvey JA, Zobitz MM, CY Pak (1988) Dose dependency of calcium absorption: a comparison of calcium carbonate and calcium citrate. J Bone Miner Res 3: 253-258.
- EG Abate, BL Clarke (2017) Review of Hypoparathyroidism. Front Endocrinol 7: 172.
- Okano K, Furukawa Y, Morii H, Fujita T (1982) Comparative efficacy of various vitamin D metabolites in the treatment of various types of hypoparathyroidism. J Clin Endocrinol Metab 55: 238-243.
- Streeten EA, Mohtasebi Y, Konig M, Davidoff L, Ryan K, et al. (2017) Hypoparathyroidism: Less severe hypocalcemia with treatment with vitamin D2 compared with calcitriol. J Clin Endocrinol Metab 102: 1505-1510.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96: 1911-1930.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, et al. (1978) Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 298: 577-581.
- Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, et al. (2003) Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone (1-34) versus calcitriol and calcium. J Clin Endocrinol Metab 88: 4214-4220.
- Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, et al. (2010) Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J Clin Endocrinol Metab 95: 2680-2688.
- Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, et al. (2012) Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97: 391-399.
- Mannstadt M, Clarke BL, Vokes T, Brandi ML, Ranganath L, et al. (2013) Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomized, phase 3 study. Lancet Diabetes Endocrinol 1: 275-283.
- Sikjaer T, Rolighed L, Hess A, Fuglsang-Fredericksen A, Mosekilde L, et al. (2014) Effects of PTH (1-84) therapy on muscle function and quality of life in hypoparathyroidism: results from a randomized controlled trial. Osteoporos Int 25: 1717-1726.
- Rubin MR, Cusano NE, Fan WW, Delgado Y, Zhang C, et al. (2016) Therapy of Hypoparathyroidism with PTH (1-84): A prospective six year investigation of efficacy and safety. J Clin Endocrinol Metab 101: 2742-2750.
- Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, et al. (2012) The US post-marketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res 27: 2429-2437.
- Agha A, Scherer MN, Moser C, Karrasch T, Girlich C, et al. (2016) Living-donor parathyroid allotransplantation for therapy-refractory postsurgical persistent hypoparathyroidism in a nontransplant recipient – 3 year results: a case report. BMC Surg 16: 51.
- Cabane P, Gac P, Amat J, Pineda P, Rossi R, et al. (2009) Allotransplant of microencapsulated parathyroid tissue in severe postsurgical hypoparathyroidism: a case report. Transplant Proc 41: 3879-3883.
- Yucesan E, Goncu B, Basoglu H, Ozten Kandas N, Ersoy YE, et al. (2017) Fresh tissue parathyroid allotransplantation with short-term immunosuppression: 1-year follow-up. Clin Transplant 31.
- Chapelle T, Meuris K, Roeyen F, De Greef K, Van Beeumen G, et al. (2009) Simultaneous kidney-parathyroid allotransplantation from a single donor after 20 years of tetany: a case report. Transplant Proc 41: 599-600.
- Garcia-Roca R, Garcia-Aroz S, Tzvetanov IG, Giulianotti PC, Campara M, et al. (2016) Simultaneous living donor kidney and parathyroid allotransplantation: first case report and review of the literature. Transplantation 100: 1318-1321.
- https://www.omim.org/
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences