Calcification of Soft Tissues in a Family, Case Report and Review of Pseudohypoparathyroidism
Navaeifar MR and Zamanfar D
DOI10.21767/2380-7245.100169
Navaeifar MR1 and Zamanfar D2*
1Pediatric Infectious Diseases Research Center, Mazandaran University of Medical Sciences, Sari, Iran
2Diabetes Research Center, Mazandaran University of Medical Sciences, Sari, IR Iran
- *Corresponding Author:
- Daniel Zamanfar
Diabetes Research Center
Mazandaran University of Medical Sciences
Sari, IR Iran
Tel: +98-1133344506
Fax: +98-1133344506
E-mail: danielzamanfar@yahoo.com
Received Date: August 05, 2017; Accepted Date: November 23, 2017; Published Date: November 30, 2017
Citation: Navaeifar MR, Zamanfar D (2017) Calcification of Soft Tissues in a Family, Case Report and Review of Pseudohypoparathyroidism. J Rare Disord Diagn Ther 3:16. doi: 10.21767/2380-7245.100169
Abstract
Pseudohypoparathyroidism and pseudo-pseudohypoparathyroidism are rare genetic disorders of calcium metabolism and resistance to the action of Parathormone. Although the genetic details of these conditions are not fully diagnosed, role of gene coding for stimulatory G protein locus was studied and imprinting in its inheritance was proved. We report three cases of a family member with calcification in soft tissues that show Pseudohypoparathyroidism in father and his sister and pseudo-pseudohypoparathyroidism in his son.
Keywords
Pseudohypoparathyroidism; Pseudopseudohypoparathyroidism; GTP-Binding Protein alpha Subunits; Bone Diseases
Introduction
A rare genetic disorder of calcium metabolism is pseudohypoparathyroidism (PHP). Pseudo-pseudohypoparathyroidism (PPHP; OMIM #612463) is the more rare disorder of calcium metabolism [1]. These conditions could be seen separately or concomitant together in kindred [2]. They also variably accompanying with Albright hereditary osteodystrophy (AHO) that described by Albright et al. as the first hormone resistance syndrome [3-5]. They reported three cases with hypocalcemia and hyperphosphatemia with normal renal function tests. They had highly resistance to bovine parathyroid extract, introduced hypothesis of resistant to Parathormone (PTH) effect.
PHP refers to a heterogeneous group of resistance to the action of PTH and it is suspected by hypocalcemia despite high serum levels of PTH [6,7]. Symptoms of hypocalcemia such as tetany and seizure could be present or absent. Basal ganglia calcification and cataract might be concomitant finding in PHP. Usually, the PHP classified based on clinical features, biochemical signs, response to exogenous PTH and genetic evaluation [6]. Although recently a novel classification proposed by the EuroPHP network [8].
Case Presentation
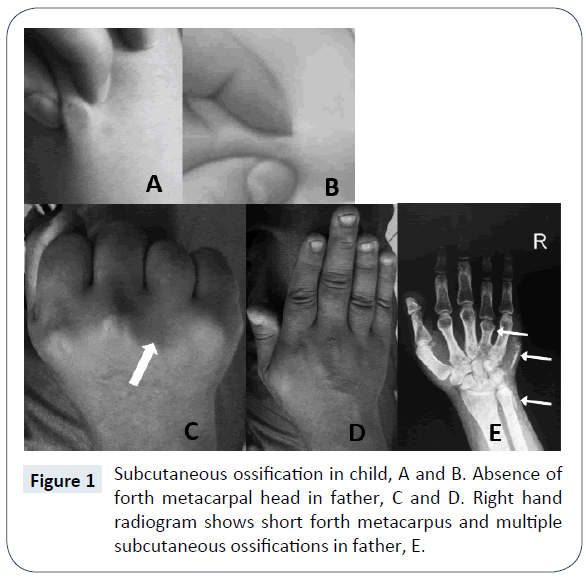
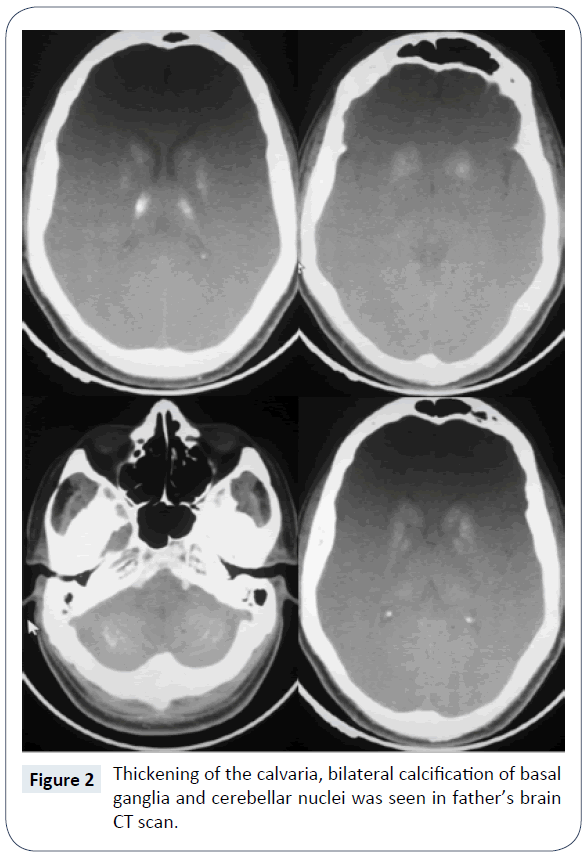
In a routine visit of a 17 month old boy, asymptomatic multiple subcutaneous calcifications in trunk, limb and neck, which was sized maximum one centimeter in diameter were founded (Figure 1). The lesions were painless and they didn’t have history of trauma. The patient was born by cesarean section on 33 weeks of gestational age and his birth weight was 1650 grams. The preterm labor was because of premature rupture of membrane. He was admitted at birth due to respiratory distress and meconium aspiration syndrome. He had an acceptable hospitalization course and discharged after 10 days. Primary laboratory evaluations at 17 months age demonstrated normal total calcium (9.7 mg/ dL), phosphorus (5.2 mg/dL), Alkaline phosphatase (733 U/L, in appropriate range for age), 25-OH Vitamin D (49 ng/ml, in appropriate range), PTH (24 pg/ml, in appropriate range), normal electrolyte and kidney function tests but a mild normochrome normocytic anemia (Hemoglobin 11 g/dL). Weight and height were under 1st percentile and head circumference was at 25th percentile, although, his socio-neurological development was mildly delayed in gross motor. The family history clarified that his father had extensive and large subcutaneous calcification especially on forearm, hand, abdominal wall, back and neck. After physical examination found that the father had short stature (1-3th percentile for age), shortening of forth metacarpal on both hands which were seen on clenched fist, loss of multiple teeth, stocky round face, brachydactyly, slight mental retardation and history of two times seizure in the past years. The patient’s paternal aunt had similar objective finding similar to patient’s father but she didn’t agree to involve in our assessment. The parents was non-consanguineous and the mother had not the same problems and was normal in physical examination and anthropometric evaluation. The other 3.5 year old boy of this parent had not objective specific finding. In evaluation of 33 year old father, low total calcium level (6.9 mg/dL), high phosphorus (5.5 mg/dL), normal Alkaline phosphatase (277 U/L), very high PTH (650 pg/ml), normal kidney and thyroid function tests were found. The 25-OH-Vit D was in mid-normal range and ultrasound evaluation of kidneys do not demonstrate significant pathology. The brain CT scan showed calcification of basal ganglia and cerebellum nuclei (Figure 2).
According to clinical and para-clinical findings, such as hypocalcemia, hyperphosphatemia, elevated PTH, shortening of metacarpus, facial sign and subcutaneous calcification, the father’s disease is a classic form of PHP type I (a or c) with AHO features but without obvious other evaluated hormone resistance. Differential diagnosis for subcutaneous ossification in our target child were: dystrophic soft tissue calcification, causing chondro-calcinosis, Fibrodysplasia ossificans progressive, posttraumatic osteoma cutis, metastatic calcification, idiopathic tumoural calcinosis, scleroderma, dermatomyositis, cysticercosis, myositis ossificans and soft tissue sarcoma. These wide range of differential diagnosis from trauma to malignancy were simply narrowed when his father’s observed after a simple family history taking! Multiple subcutaneous calcifications in a child when his father shows a classic form of PHP, strike PPHP in the mind because of its genetic nature. As we discuss it in the next section, in a family with history of PHP when the father is the origin of gene, its imprinting led to PPHP in child. When a child has PPHP, and the father shows PHP features, the father’s type of PHP is Ia. In the absence of genetic study and evaluation of urine cAMP after synthetic PTH administration it is difficult to say our diagnosis is definite. According to European PHP network classification for PHP the father case has two major criteria and is a case of “inactivating PTH/PTHrP signaling disorder” (iPPSD) without genetic study.
Pathophysiology
Because of critical function of calcium ion in much vital enzymatic process and electrical activity of neuron, muscle conductivity and contractility and many other well diagnosed functions, calcium adjustment in body fluid and cells tightly regulate by multiple organ interactions [9]. Hypocalcemia evokes parathyroid glands to increase parathyroid hormone (PTH). Parathyroid chief cells can synthesize, process, store PTH and rapidly secrete stored hormone, in addition, these cells can replicate when chronically stimulated [10]. Minutely, PTH controls the ionized calcium level of intra and extra cellular fluid. PTH directly triggers calcium elevating responses on bone and renal cells and indirectly increase intestinal calcium absorption along with bone and kidney calcium fluxes into blood, induced by increased 1, 25 (OH)2 Vit. D3 as another renal response to PTH. The major stimulator of PTH secretion is decreased ionized calcium. Moreover, Phosphate, magnesium, catecholamines, and other stimuli can partly affect PTH secretion [10-12]. Severe hypomagnesemia could reduce secretion of PTH and increase target organ resistance [13]. Brief main action of PTH on kidneys is stimulation of calcium reabsorption, Inhibition of phosphate transport and stimulates the synthesis of 1,25(OH)2 D3 [14-16] hallmark of PHP is impairment of renal action of PTH. Although, PTH effect in the thick ascending tubule seems unimpaired, resistance to PTH in the proximal tubule occur [17]. Effect of PTH on bone is correlated to hemostatic state of calcium, type of bone and type or duration of PTH administration. Across the board, PTH increase the bone resorption [18,19].
Gene role
The role of genes in forming of parathyroid cells is partly diagnosed, as well as its roles in parathyroid function and target organ response. Combination of PTH and PTH related peptide (PTHrP) activate PTH receptor (PTH1R) that lead to activating the guanine-binding proteins (G protein). Heterotrimeric G proteins are composed of three subunits and are crucial mediators of signal transduction pathways of more than 1000 G protein coupled receptors [20]. The stimulatory GTP-Binding Protein alpha Subunits (Gsα) triggers the activation of the adenylate cyclase (cAMP) leading to specific cellular functions of PTH. Studies have shown significant roles of hoxa3, pax9, GATA3, gcm2 and GCMB genes in producing and function of parathyroid glands [21-25].
An alteration of the gene coding for Gsa (GNAS) locus causes PHP Ia and PHP Ib [26]. GNAS location is on the long arm of chromosome 20 (20q13.3); the very structurally similar murine homolog is on chromosome 2 [27]. The Gsα is the most abundant and best characterized gene product that encoded by exons 1–13 [28]. Although, inheriting the heterozygous gene encoding the subunit of heterotrimeric Gsα, from the mother lead to PHP Ia, when the same gene inherited from the father lead to PPHP (Figure 1). The Gsα is an essential expressed gene in about all cells and has main roles in many of physiological processes (Figure 2). This essential role is because of its presentation in downstream of many different G protein-coupled hormone receptors [5,29]. Gsα has parent-specific imprinting that discussed later. GNAS gene has extensively influence on chondrocytes maturation and its damages could led to defect in growth palate including brachydactyly and Madelung deformity [30]. Recent studies have shown that GNAS mutations involved exons 2 through 13 reduces the birth weight of patients while patients had mutations in exon1/intron 1 have had higher birth parameter, suggesting the role of GNAS in fetal development [31]. Although 70% of patients with PHP Ia and in their sibs affected with PPHP have GNAS mutations, in the remain cases the molecular evaluations fail to show definite mutation [5].
Recently, in a large series of 40 patients, Mantovani et al. show that more than one half of patients that previously clinically diagnosed as PHP Ia but have not mutations in Gsα-coding exons, substantially, could be classified as sporadic PHP-Ib after molecular study [32]. Nanclares et al. in 2006 investigate 4 unrelated patients who have mild AHO features, TSH and PTH resistance were thought to have PHP Ia [33] they suggest that some overlap may exist between PHP Ia and PHP Ib in molecular and clinical features. This means, some patients with molecular finding of PHP Ib may have AHO feature, unexpectedly. Mariot et al. report a female case of AHO and PTH resistance without GNAS Loss-of-Function mutation [34] this indicates heterogeneity in PHP Ib and these patients may have AHO feature. Unluturk et al. describe a case of clinically diagnosed PHP Ib with favorite genetic mutation but mild AHO feature including short fourth metacarpals, round face and slightly short stature [35] this mutation also was seen in her affected sister without AHO feature and her unaffected mother. In addition, the index case and her sister have increased fractional excretion of uric acid and decreased serum uric acid level that normalized after 3 months of treatment for hypocalcemia.
Classification of PHP
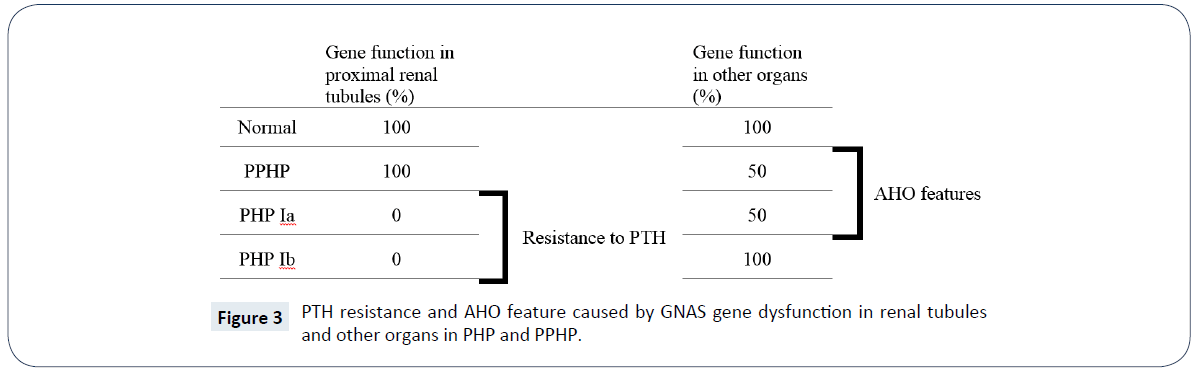
Resistance to PTH in idiopathic and inherited forms called Pseudohypoparathyroidism. Hypocalcemia and hyperphosphatemia is present in classic PHP and phosphate diuresis or elevation in calcium are not happen after administration of exogenous PTH [36,37]. Several forms described for PHP, The two main subtypes are type Ia and Ib, other types are Ic and type 2. Some patients in the family of PHP genetically have same mutation in GNAS1 and phenotypically have AHO feature but have not resistant to PTH that called PPHP [38]. Usually, PPHP classified as a subtype of PHP because of its genetic correspondence, see (Table 1 and Figure 3). According to Orphanet reports, prevalence of PHP (including types Ia and Ib) and PHP (including types Ia, Ib and PPHP), has been estimated at 1/295,000 in a Japanese study and 1/150,000 in Italy, respectively. Female were twice more affected by PHP than men [39]. In PHP I subtypes the exogenous PTH fails to increase serum calcium level or increase the urinary phosphate and cAMP secretion, due to impairment in PTH receptor coupling [26,38]. But, in PHP type II the same test as above fails to increase serum calcium level or increase the urinary phosphate secretion whereas the cAMP urinary secretion is normal, these means the defect is distal to cAMP generation point [37,40]. In PHP Ia (OMIM #103580) and PHP Ic (OMIM #612462) the feature of AHO including short stature, rounded face, foreshortened metacarpals and metatarsals, obesity [41], stocky habitus, obesity, developmental delay, dental hypoplasia, soft tissue calcification/ossification and subcutaneous calcifications are present, but in PHP type Ib (OMIM #603233) there are not [42-46]. Hypocalcemic patients may have sign and symptoms of decreased ionized calcium such as tetany or seizure [47]. Signs and symptoms that are suggest deficiencies of thyrotropin, the gonadotropins, glucagon, antidiuretic hormone, adrenocorticotropin, and growth hormone–releasing hormone may present in PHP Ia, because these peptide hormones use the alpha subunit of the Gsa protein for production of cAMP [48]. Although, men with PHP Ia may show features of hypogonadism and infertility, these conditions is less obvious in men than women. Multi-hormone resistance and presence of AHO both seen in PHP Ia and PHP Ic, but partial deficiency of Gsα activity, about 50%, is only seen in PHP Ia, see (Figure 3) [49]. Although, Mental retardation has been suggested to be an additional clinical characteristics of AHO since its first reports, but the severity and frequency of this sign are not well accepted [50,51]. Albright et al. 10 years after the first report, describe patients showing the features of AHO without any evidence of PTH resistance were also described previously [52] this syndrome called PPHP and could be found as sporadic or in known kindred of PHP. The PPHP frequently seen in family with history of PHP Ia with inheritance called genetic imprinting that in this case means, when the impaired Gsα gene inherit from father, the child exhibits PPHP, and when inherit from mother, child shows PHP manifestations and have the same percentage of Gsα activity deficiency in cell membranes [7,38,53-55].
| PHP subtype | Serum calcium | Serum P | Serum PTH | Urine cAMP after synthetic PTH administered | Hormone resistance | AHO features | GNAS defect | Activity of Gsα |
|---|---|---|---|---|---|---|---|---|
| PHP Ia | ↓ | ↑ | ↑ | ↓ | Multiple: PTH, TSH, Gn, GHRH | Yes | Maternal inactivating mutation | ↓ |
| PHP Ib | ↓ | ↑ | ↑ | ↓ | PTH, TSH | No | Imprinting dysregulation | Nl |
| PHP Ic | ↓ | ↑ | ↑ | ↓ | Multiple: PTH, TSH, Gn | Yes | Few inactivating mutation reported | Nl |
| PHP II | ↓ | ↑ | ↑ | Nl | No | - | Nl | |
| PPHP | Nl | Nl | Nl | Nl | No | Yes/no | Paternal inactivating mutations | ↓ |
Abbreviations: PHP, pseudohypoparathyroidism; PPHP, pseudo-pseudohypoparathyroidism; P, phosphorous; PTH, parathyroid hormone; cAMP, cyclic adenosine monophosphate; AHO, Albright’s hereditary osteodystrophy; Gsα, alpha subunit of the stimulatory G-protein; Nl; Normal; Gn, Gonadotropins; TSH, Thyroid stimulating hormone; GHRH, Growth-hormone-releasing hormone.
Table 1: Differentiating the multiple subtypes of PHP [6,64].
Patients suffering from PTH resistance and hypocalcemia/ osteomalacia without AHO features are classified in PHP 2 group. Genetic aspect of PHP 2 is not well known until now [56]. Progressive osseous heteroplasia (POH, OMIM #166350), inherited like PPHP from the father gene. POH characterized by heterotopic ossifications not only in subcutaneous tissue but expanding into deep muscles and connective tissues. They have not hormone resistance but have heterozygous mutation in GNAS [57]. Acrodysostosis is another AHO featured of this family. Some of them had cognitive impairment, severe AHO feature, PTH and TSH resistant and hypertension. Heterozygous mutation was found in some cases in PRKAR1A, PDE4D or PDE3A coding sequences [58-60]. Deletion of the 2q37.3 chromosomal region including HDAC4 could led to 2q37.3 deletion syndrome presenting with AHO feature and cognitive impairment/ psychosis without PTH resistance [61]. The Blomstrand chondrodysplasia (OMIM #215045) is a lethal form of dwarfism, associated with biallelic inactivating mutation of the PTH1R gene [62]. A milder form of biallelic inactivating mutation of the PTH1R gene is Eiken epiphyseal dysplasia, presented with short stature and elevated PTH in some cases [63]. Recently, European PHP network proposed a novel classification for PHP [8] they proposed the term “inactivating PTH/PTHrP signaling disorder” (iPPSD) instead of pseudohypoparathyroidism. The iPPSD classification recommended the following categories: iPPSD: clinical/biochemical diagnosis based on the major/minor criteria (Table 2), in the absence of genetic investigation; this category is recommended for individuals with a pure clinical suspicion of iPPSD and lack of complete (epi) genetic testing.
| Assessment | Differential diagnosis | ||
|---|---|---|---|
| Major criteria | •PTH resistance | Ionized calcium, total calcium | Normocalcemic |
| Phosphate | hyperparathyroidism | ||
| Magnesium | Renal failure | ||
| PTH | Vitamin D deficiency or | ||
| Vitamin D (25OHD) | any kind of secondary | ||
| Creatinine | hyperparathyroidism | ||
| Urinary calcium | |||
| Urinary phosphate | |||
| PTH infusion test in challenging | |||
| cases | |||
| •Ectopic ossification | Detailed physical exam | Fibrodysplasia ossificans | |
| X-rays | progressiva (FOP, OMIM #135100), post-traumatic osteoma cutis | ||
| •Brachydactyly type E | Clinical inspection (fist), hand and | Turner syndrome, | |
| (comprises the IV) | feet X-rays | tricho-rhino-phalangeal | |
| syndrome (TRPS), TRPS I, (OMIM #190350), | |||
| TRPS-II (OMIM #150230) | |||
| and TRPS-III, (OMIM | |||
| #190351) | |||
| Minor criteria | •TSH resistance | TSH, T4l, antibodies, imaging† | Mutations in the TSH |
| receptor | |||
| •Other hormonal | IGF-1 (GH stimulation test if | ||
| resistances | necessary), calcitonin, LH, FSH, | ||
| GnRH test | |||
| •Motor and cognitive | Computed tomography scan and/or | ||
| retardation or | MRI of the brain, psychopathological | ||
| impairment | rating scales adjusted | ||
| for age | |||
| •Intrauterine and | IUGR: gestational age, birth weight, birth length, head circumference, comparison to reference charts; post-natal growth: growth charts, X-ray of the left hand for determination of the bone age | ||
| postnatal growth | |||
| retardation | |||
| •Obesity/overweight | Weight SDS, BMI percentile, BMI | ||
| z-score | |||
| •Flat nasal bridge | Clinical inspection | ||
| and/or maxillar | |||
| hypoplasia and/or | |||
| round face | |||
| iPPSD clinical diagnosis | (a) Presence of one major criteria, either number 1 or 2; | ||
| (b) Presence of major criteria number 3 and at least 2 minor criteria‡ | |||
| †US in adults with hypothyroidism and no evidence for autoimmunity; thyroid imaging through thyroid scintigraphy and US in neonates diagnosed through screening for congenital hypothyroidism; | |||
| ‡Minor criteria are nonspecific (obesity/cognitive impairment); for instance, the association of BDE + obesity or BDE + cognitive impairment would not be relevant for our classification. By raising the number of minor criteria from 1 to 2, we will reduce the risk of overdiagnosing patients with iPPSD. | |||
| From: Thiele S, et al. Pseudohypoparathyroidism to inactivating PTH/PTHrP signalling disorder (iPPSD), a novel classification proposed by the EuroPHP network. European Journal of Endocrinology. 2016;175(6):P1-P17. | |||
Table 2: Definition of major and minor criteria for iPPSD and differential diagnoses [8].
iPPSD1: loss-of-function mutation in PTH1R.
iPPSD2: loss-of-function mutation in GNAS Gsa sequence.
iPPSD3: methylation change(s) at one or more GNAS DMRs, associated with or without a genetic (deletion) or cytogenetic (UPD) defect.
iPPSD4: PRKAR1A mutation.
iPPSD5: PDE4D mutation.
iPPSD6: PDE3A mutation.
iPPSDx: lack of genetic/epigenetic defect identified following molecular investigation of known genes described above.
iPPSDn+1: the identification of a novel gene/molecular defect will lead to a disease named iPPSD7, then 8 and so on.
They believe that this new nomenclature will facilitate approach to the diagnosis of iPPSD and it would allow for the classification of patients in a more homogenous way and lead to better future observational and research studies in the field.
Conclusion
Although clinical presentation of PHP and PPHP was known and multiple organ involvement was explained in detail, the genetic and molecular pattern of this condition was not clearly distinct and investigations are in progress. Despite the fact that according to clinical feature and simple laboratory tests, our index case has PPHP and his father and fathers’ sister are cases of PHP Ia, but in lack of genetic study the autosomal dominant PHP Ib could not be rollout in patients’ aunt [35].
Typical AHO feature, hypocalcemia, hyperphosphatemia and high PTH of father in addition to PPHP phenotype in his child, that is compatible with paternal imprinting, affirm the diagnosis of PHP Ia for father.
References
- Elli FM, deSanctis L, Ceoloni B, Barbieri AM, Bordogna P, et al. (2013) Pseudohypoparathyroidism type Ia and pseudo-pseudohypoparathyroidism: the growing spectrum of GNAS inactivating mutations. Hum Mutat 34: 411-416.
- Thiele S, Werner R, Grötzinger J, Brix B, Staedt P, et al. (2015) A positive genotype–phenotype correlation in a large cohort of patients with Pseudohypoparathyroidism Type Ia and Pseudopseudohypoparathyroidism and 33 newly identified mutations in the GNAS gene. Mol Genet Genomic Med 3: 111-120.
- Albright F, Burnett CH, Smith PH (1942) Pseudo-hypoparathyroidism-example of 'Seabright-Bantam syndrome'; report of three cases. Endocrinology 30: 922-932.
- Mantovani G, Spada A (2006) Mutations in the Gs alpha gene causing hormone resistance. Best Pract Res Clin Endocrinol Metab 20: 501-513.
- Weinstein LS, Yu S, Warner DR, Liu J (2001) Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev 22: n675-705.
- Mantovani G (2011) Clinical review: Pseudohypoparathyroidism: diagnosis and treatment. J Clin Endocrinol Metab 96: 3020-3030.
- Turan S, Bastepe M (2013) The GNAS complex locus and human diseases associated with loss-of-function mutations or epimutations within this imprinted gene. Horm Res Paediatr 80: 229-241.
- Thiele S, Mantovani G, Barlier A, Boldrin V, Bordogna P, et al. (2016) From pseudohypoparathyroidism to inactivating PTH/PTHrP signalling disorder (iPPSD), a novel classification proposed by the EuroPHP network. Eur J Endocrinol 175: P1-P17.
- Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM, et al. (2011) Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc Natl Acad Sci U S A 108: 191-196.
- Brown E (1982) PTH secretion in vivo and in vitro. Regulation by calcium and other secretagogues. Miner Elecrolyte Metab 8: 130.
- Estepa JC, Aguilera‐Tejero E, Lopez I, Almaden Y, Rodriguez M, et al. (1999) Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res 14: 1848-1854.
- Kilav R, Silver J, Naveh-Many T (1995) Parathyroid hormone gene expression in hypophosphatemic rats. J Clin Invest 96: 327.
- Suh SM, Tashjian Jr AH, Matsuo N, Parkinson DK, Fraser D, et al. (1973) Pathogenesis of hypocalcemia in primary hypomagnesemia: normal end-organ responsiveness to parathyroid hormone, impaired parathyroid gland function. J Clin Invest 52: 153.
- Friedman PA, Gesek FA (1995) Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiol Rev 75: 429-471.
- Murayama A, Takeyama K-i, Kitanaka S, Kodera Y, Kawaguchi Y, et al. (1999) Positive and Negative Regulations of the Renal 25-Hydroxyvitamin D3 1α-Hydroxylase Gene by Parathyroid Hormone, Calcitonin, and 1α, 25 (OH) 2D3 in Intact Animals 1. Endocrinology 140: 2224-2231.
- Shimizu T, Yoshitomi K, Nakamura M, Imai M (1990) Effect of parathyroid hormone on the connecting tubule from the rabbit kidney: biphasic response of transmural voltage. Pflügers Archiv 416: 254-261.
- Stone M, Hosking D, Garcia-Himmelstine C, White D, Rosenblum D, et al. (1993) The renal response to exogenous parathyroid hormone in treated pseudohypoparathyroidism. Bone 14: 727-735.
- Canalis E, Centrella M, Burch W, McCarthy TL (1989) Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83: 60.
- Ishizuya T, Yokose S, Hori M, Noda T, Suda T, et al. (1997) Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99: 2961.
- Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85: 1159-1204.
- BERSON AS, YALOW RS (1968) Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab 28: 1037-1047.
- Ding C, Buckingham B, Levine MA (2001) Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest 108: 1215.
- Günther T, Chen ZF, Kim J, Priemel M, Rueger JM, et al. (2000) Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 406: 199-203.
- Manley NR, Capecchi MR (1998) HoxGroup 3 Paralogs Regulate the Development and Migration of the Thymus, Thyroid, and Parathyroid Glands. Dev Biol 195: 1-15.
- Peters H, Neubüser A, Kratochwil K, Balling R (1998) Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 12: 2735-2747.
- Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ (2015) PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol 11: 712-724.
- Tafaj O, Jüppner H (2016) Pseudohypoparathyroidism: one gene, several syndromes. J Endocrinol Invest 40: 347-356.
- Kozasa T, Itoh H, Tsukamoto T, Kaziro Y (1988) Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci 85: 2081-2085.
- Bastepe M, Jüppner H (2000) Pseudohypoparathyroidism: new insights into an old disease. Endocrinol Metab Clin North Am 29: 569-589.
- Sanchez J, Perera E, Jan de Beur S, Ding C, Dang A, et al. (2011) Madelung-like deformity in pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab 96: E1507-E1511.
- Richard N, Molin A, Coudray N, Rault-Guillaume P, Jüppner H, et al. (2013) Paternal GNAS mutations lead to severe intrauterine growth retardation (IUGR) and provide evidence for a role of XLαs in fetal development. J Clin Endocrinol Metab 98: E1549-E1556.
- Mantovani G, de Sanctis L, Barbieri AM, Elli FM, Bollati V, et al. (2010) Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab 95: 651-658.
- de Nanclares GP, Fernández-Rebollo E, Santin I, García-Cuartero B, Gaztambide S, et al. (2007) Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab 92: 2370-2373.
- Mariot V, Maupetit-Méhouas S, Sinding C, Kottler ML, Linglart A, et al. (2008) A maternal epimutation of GNAS leads to Albright osteodystrophy and parathyroid hormone resistance. J Clin Endocrinol Metab 93: 661-665.
- Unluturk U, Harmanci A, Babaoglu M, Yasar U, Varli K, et al. (2008) Molecular diagnosis and clinical characterization of pseudohypoparathyroidism type-Ib in a patient with mild Albright's hereditary osteodystrophy-like features, epileptic seizures, and defective renal handling of uric acid. Am J Med Sci 336: 84-90.
- Carter A, Bardin C, Collins R, Simons C, Bray P, et al. (1987) Reduced expression of multiple forms of the alpha subunit of the stimulatory GTP-binding protein in pseudohypoparathyroidism type Ia. Proc Natl Acad Sci 84: 7266-7269.
- Chase LR, Melson GL, Aurbach G (1969) Pseudohypoparathyroidism: defective excretion of 3′, 5′-AMP in response to parathyroid hormone. J Clin Invest 48: 1832.
- Bastepe M (2008) The GNAS locus and pseudohypoparathyroidism. Genomic imprinting. Springer pp: 27-40.
- Mantovani G (2014) Pseudohypoparathyroidism type 1A.
- Drezner M, Neelon FA, Lebovitz HE (1973) Pseudohypoparathyroidism type II: a possible defect in the reception of the cyclic AMP signal. N Engl J Med 289: 1056-1060.
- Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL, et al. (2007) Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gαs in the development of human obesity. J Clin Endocrinol Metab 92: 1073-1079.
- Caroline S, Santora A, Breslau N, Moses A, Spiegel A, et al. (1986) Selective Resistance to Parathyroid Hormone in Cultured Skin Fibroblasts from Patients with Pseudohypoparathyroidism Type Ib*. J Clin Endocrinol Metab 62: 640-644.
- Farfel Z, Brickman AS, Kaslow HR, Brothers VM, Bourne HR, et al. (1980) Defect of receptor–cyclase coupling protein in pseudohypoparathyroidism. N Engl J Med 303: 237-242.
- Levine M, Downs R, Singer M, Marx S, Aurbach G, et al. (1980) Deficient activity of guanine nucleotide regulatory protein in erythrocytes from patients with pseudohypoparathyroidism. Biochem Biophys Res Commun 94: 1319-1324.
- Levine MA, Downs RW, Moses AM, Breslau NA, Marx SJ, et al. (1983) Resistance to multiple hormones in patients with pseudohypoparathyroidism: association with deficient activity of guanine nucleotide regulatory protein. Am J Med 74: 545-556.
- Radeke HH, Aufmkolk B, Jüppner H, Krohn H-P, Keck E, et al. (1986) Multiple Pre-and Postreceptor Defects in Pseudohypoparathyroidism (A Multicenter Study with Twenty Four Patients)*. J Clin Endocrinol Metab 62: 393-402.
- Ritter C, Göbel CH, Liebig T, Kaminksy E, Fink GR, et al. (2015) An epigenetic cause of seizures and brain calcification: pseudohypoparathyroidism. The Lancet 385: 1802.
- Balavoine A-S, Ladsous M, Velayoudom F-L, Vlaeminck V, Cardot-Bauters C, et al. (2008) Hypothyroidism in patients with pseudohypoparathyroidism type Ia: clinical evidence of resistance to TSH and TRH. Eur J Endocrinol 159: 431-437.
- Brix B, Werner R, Staedt P, Struve D, Hiort O, et al. (2014) Different pattern of epigenetic changes of the GNAS gene locus in patients with pseudohypoparathyroidism type Ic confirm the heterogeneity of underlying pathomechanisms in this subgroup of pseudohypoparathyroidism and the demand for a new classification of GNAS-related disorders. J Clin Endocrinol Metab 99: 2013-4477.
- Farfel Z, FRIEDMAN E (1986) Mental deficiency in pseudohypoparathyroidism type I is associated with Ns-protein deficiency. Ann Intern Med 105: 197-199.
- Wilson LC, Trembath RC (1994) Albright's hereditary osteodystrophy. J Med Genet 31: 779-784.
- Albright F, Forbes AP, Henneman P (1952) Pseudo-pseudohypoparathyroidism. Transactions of the Association of Am Physicians 65: 337.
- Davies SJ, Hughes HE (1993) Imprinting in Albright's hereditary osteodystrophy. J Med Genet 30: 101-103.
- Turan S, Thiele S, Tafaj O, Brix B, Atay Z, et al. (2015) Evidence of hormone resistance in a pseudo-pseudohypoparathyroidism patient with a novel paternal mutation in GNAS. Bone 71: 53-57.
- Wilson LC, Luttikhuis MO, Clayton PT, Fraser WD, Trembath R, et al. (1994) Parental origin of Gs alpha gene mutations in Albright's hereditary osteodystrophy. J Med Genet 31: 835-839.
- Rao DS, Parfitt AM, Kleerekoper M, Pumo BS, Frame B, et al. (1985) Dissociation between the effects of endogenous parathyroid hormone on adenosine 3', 5'-monophosphate generation and phosphate reabsorption in hypocalcemia due to vitamin D depletion: an acquired disorder resembling pseudohypoparathyroidism type II. J Clin Endocrinol Metab 61: 285-290.
- Lebrun M, Richard N, Abeguile G, David A, Coeslier Dieux A, et al. (2010) Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab 95: 3028-3038.
- Linglart A, Menguy C, Couvineau A, Auzan C, Gunes Y, et al. (2011) Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med 364: 2218-2226.
- Maass PG, Aydin A, Luft FC, Schachterle C, Weise A, et al. (2015) PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat Genet 47: 647-653.
- Nagasaki K, Iida T, Sato H, Ogawa Y, Kikuchi T, et al. (2012) PRKAR1A mutation affecting cAMP-mediated G protein-coupled receptor signaling in a patient with acrodysostosis and hormone resistance. J Clin Endocrinol Metab 97: 2012-1369.
- Mehraein Y, Pfob M, Steinlein O, Aichinger E, Eggert M, et al. (2015) 2q37.3 Deletion Syndrome: Two Cases with Highly Distinctive Facial Phenotype, Discordant Association with Schizophrenic Psychosis, and Shared Deletion Breakpoint Region on 2q37.3. Cytogenet Genome Res 146: 33-38.
- Hoogendam J, Farih-Sips H, Wynaendts LC, Lowik CW, Wit JM, et al. (2007) Novel mutations in the parathyroid hormone (PTH)/PTH-related peptide receptor type 1 causing Blomstrand osteochondrodysplasia types I and II. J Clin Endocrinol Metab 92: 1088-1095.
- Duchatelet S, Ostergaard E, Cortes D, Lemainque A, Julier C, et al. (2005) Recessive mutations in PTHR1 cause contrasting skeletal dysplasias in Eiken and Blomstrand syndromes. Hum Mol Genet 14: 1-5.
- Raghavan P, Katz CM (2012) Pseudohypoparathyroidism type Ia manifesting as intractable epilepsy in a 23-year-old female. Int Med Case Rep J 5: 49.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences