A Rare Case of an Intrapancreatic Gastric Duplication Cyst as the Cause of Recurrent Pancreatitis. Literature Review of Gastric Duplication Cysts in Adult Population
Konstantinos S Flamourakis1*, Nikolaos Memos1, Dimitrios P Bouklas2, Despoina Myoteri2, Manousos M Konstadoulakis1 and Dina G Tiniakos2
1Department of Surgery, Aretaieion University Hospital, National Kapodistrian University of Athens (NKUA), Athens, Greece
2Department of Pathology, Aretaieion Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
*Corresponding Author:
- Konstantinos S Flamourakis,Department of Surgery, Aretaieion University Hospital, National Kapodistrian University of Athens (NKUA), Athens, Greece, E-mail:kflamourakis@gmail.com
Received date: May 24, 2022, Manuscript No. IPRDDT-22-12510; Editor Assigned date: May 26, 2022, PreQC No. IPRDDT-22-12510(PQ); Reviewed date: June 09, 2022, QC No IPRDDT-22-12510; Revised date:June 17, 2022, Manuscript No. IPRDDT-22-12510(R); Published date: June 27, 2022, DOI: 10.36648/2380-7245.8.6.43
Citation:Flamourakis KS, Memos N, Bouklas DP, Myoteri D, Konstadoulakis MM, et al. (2022). A Rare Case of an Intrapancreatic Gastric Duplication Cyst as the Cause of Recurrent Pancreatitis. Literature Review of Gastric Duplication Cysts in Adult Population. J Rare Disord Diagn Ther Vol.8 No.6: 43
Abstract
Duplication cysts of the Gastrointestinal (GI) tract are a rare congenital disease usually presenting in childhood. They may be located at any level of the GI tract but are more frequent in the ileum, followed by the esophagus, the duodenum and stomach. Gastric Duplication Cysts (GDC) account for approximately 4%-8% of all GI duplication cysts. We herein present a case of an 18-year-old male with familiar hypertriglyceridemia who was diagnosed with a 17 cm benign intrapancreatic GDC etiologically related to his recurrent pancreatitis since the age of 9 years old. Magnetic resonance imaging (T1, T2, and DWI weighted), Magnetic resonance cholangiopancreatography and computed tomography were utilized for the preoperative evaluation which showed a non-communicating gastric duplication cyst surrounded by the pancreatic tail and the spleen. The diagnosis was based on histopathological examination of the resected specimen.
Keywords:Keywords: Gastric duplication cyst; Foregut duplication cyst; Intrapancreatic gastric duplication cyst; Intrapancreatic enteric duplication cyst; Recurrent pancreatitis
Introduction
Duplication Cysts can be found at any level of the GI tract, from the oral cavity to the rectum. According to the most prevalent theory about their origin, abnormal vacuolization or recanalization of the intestinal tract during embryonic development following the solid epithelial stage appears to be the plausible cause for these cystic malformations. Associated congenital anomalies such as alimentary tract duplications, esophageal diverticula or spinal cord abnormalities are encountered in up to 50% of these patients [1]. Enteric duplication cysts can be cystic (non-communicating with the stomach) or tubular (in direct communication with the stomach lumen). The cystic formations are usually identified in the neonatal period, when they present with gastrointestinal symptoms, such as vomiting, abdominal pain, discomfort or weight loss. The essential criteria for a cystic formation to be diagnosed as a Gastric Duplication Cyst (GDC) are described in Table 1. Over 70% of GDC have been reported at the age of 12 and below [2,3]. Adults remain in most cases asymptomatic and the cystic lesion is found incidentally after a Computed Tomography (CT), Magnetic Resonance Imaging (MRI) or Endoscopic Ultrasonography (EUS) scan. In some cases, the cyst may induce mild symptoms, such as abdominal discomfort or early satiation, and, in rarer cases, severe gastrointestinal symptoms, i.e. hematemesis and melena [4]. We report a rare case of intrapancreatic non-communicating GDC diagnosed postoperatively and review the related literature highlighting the diagnostic difficulty and optimal therapeutic approach (Table 1).
| Cyst wall is contiguous with the wall of the stomach. |
| Cyst wall contains smooth muscle continuous with the gastric wall muscle layer. |
| Cyst wall is lined by gastric or gut mucosa (when lining is not necrotic) |
Table 1: Essential criteria for diagnosis of a gastric duplication cyst. Data collected from literature, especially from Passos et al. [1] Singh et al. [5] Fukomoto et al [6] and Kuraoka et al. [7]
Case Presentation
The patient is an 18-year-old male, product of a twin birth, presented with recurrent episodes of non-radiating epigastric pain, nausea, emesis and fever since the age of 9 years old. The initial diagnosis was recurrent pancreatitis with pseudocyst formation due to Familiar Hypertriglyceridemia (triglycerides>1000 mg/dl). The patient presented to our surgical department approximately one week after the latest onset of sharp epigastric pain similar to that of acute pancreatitis, nausea and fever. There were no changes in bowel habits. The patient denied coffee, alcohol and tobacco consumption, emesis, hematemesis, melena or weight loss.
Patient’s history revealed multiple episodes of acute pancreatitis and multiple hospitalizations since the age of 9 years with serum amylase levels >5.000 IU. In 2018, he was hospitalized with the diagnosis of pancreatic pseudocyst (17 cm in diameter). An endoscopic drainage and stent placement was performed. The stent was removed a month later, as the diameter of the cyst reduced to 1.5 cm.
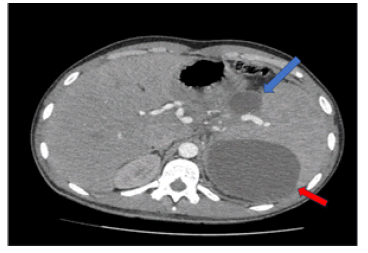
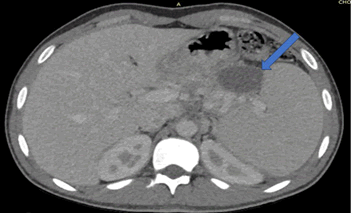
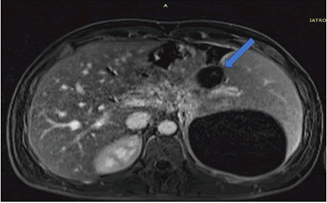
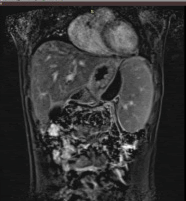
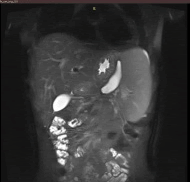
Following initial treatment, the epigastric pain did not resolve and he was treated with plasmapheresis as his triglyceride levels were not controlled by the medical treatment. On the follow up scans, the patient was then evaluated with MRI (T1, T2 and DWI weighted), Magnetic Resonance Cholangiopancreatography (MRCP) and Computed Tomography CT with contrast medium. Imaging revealed an intrapancreatic cyst-like lesion extending from the pancreatic body toward the gastrosplenic ligament near the greater curvature of the gastric body and measuring 6.9 cm × 3 cm, at the same site as the initial pancreatic pseudocyst. Furthermore, splenomegaly and two splenic cysts with diameters of 10. × 9.4 cm and 10.6 × 8.6 cm were observed (Figures 1-6).
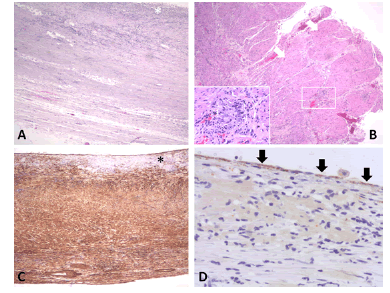
Figure 2: Cystic wall covered by amorphous material (white asterisk) overlying smooth muscle layers (haematoxylin & eosin- H&E, x40). B. Cyst wall with two distinct muscle layers (white arrows) and myenteric plexus (white rectangle) in between these (H&E, x40). Inset shows the myenteric ganglion depicted within the white rectangle in higher magnification (H&E, x200). C. Immunostain with alpha- smooth muscle actin highlights the muscle wall of the cyst. The amorphous material (black asterisk) covering the inner surface is negative (3’,3’diaminobenzidine chromagen, x40). D. Immunostain with pankeratin highlights rare epithelial cells (black arrows) on the inner surface of the cyst (3’,3’diaminobenzidine chromagen, x100).
Due to the epigastralgia and the failure of conservative measures to control pseudocyst formation, surgical treatment was decided. The patient subsequently underwent an exploratory laparotomy during which the presence of the cystic lesion along the gastrosplenic ligament and originating from the pancreatic tail and body was confirmed. A distal pancreatectomy, splenectomy and cholecystectomy were performed.
On gross pathology examination of the peripheral pancreatectomy and splenectomy specimen a large cystic lesion occupying part of the spleen and extending to the adjacent resected pancreatic tail was seen. On sectioning, the cyst was bilocular with wall thickness 0.1 cm-0.4 cm. The two communicating cystic spaces measured 9 cm and 10 cm in maximal diameter, respectively and their internal surface was smooth and focally granular. The cystic cavities contained amorphous soft brownish material. On histology, their internal surface was covered by amorphous necrotic tissue overlying a smooth muscle wall with two distinct layers resembling those of the gastrointestinal wall. In between the inner circular and outer longitudinal smooth muscle layers, rare neural plexus features with ganglion cells were observed. Immunohistochemistry for α-smooth muscle actin and desmin, pankeratin and CD31 confirmed the presence of smooth muscle layers, rare covering epithelial cells, and absence of endothelial lining, respectively. The excised pancreatic tail showed exocrine parenchymal atrophy, fibrosis and chronic inflammatory changes, while the spleen showed white pulp atrophy. The overall histology in conjunction with the anatomical location and the clinical history supported the diagnosis of non-communicating gastric duplication cyst surrounded by the pancreatic tail and the spleen. There was no evidence of epithelial dysplasia or malignancy.
Postoperative course was uneventful and the patient was discharged on the 6th postoperative day. He has been asymptomatic to date, 1.5 year after the diagnosis.
Discussion
Gastrointestinal Duplication Cysts (GDC) is uncommon congenital malformations that occur at any level from the oral cavity to the rectum with the ileum being the most common site. CDCs are extremely rare, accounting for 4%-8% of all gastrointestinal duplications [8-10]. There is no significant gender predilection (55% females), and most cases are diagnosed in the pediatric or young adult population [3]. In older individuals, the mean age of diagnosis is 45 years old and, in most cases the cyst is solitary while rarely up to four cystic lesions have been reported. The mean maximum diameter of the cysts is 5,5 cm (1 cm-25 cm).
Intrapancreatic CDC originate from the adjacent GI tract during midgut differentiation [11], then become embedded in the pancreas, and eventually separate from the GI tract. Later in life, these cysts fill with secretions, enlarge and erode into the pancreatic duct becoming symptomatic.
Traverso et al. noted that patients in whom the gastric duplication cyst was continuous to the stomach had recurrent episodes of pancreatitis. The mechanical cause of pancreatitis was thought to be obstructive due to cystic secretions or blood coagula and debris from cystic ulcerations. The rarity of this malformation is responsible for the incorrect preoperative diagnosis in most cases, sometimes resulting in multiple operative explorations.
GDC is often associated with other congenital malformations, such as pancreatic heterotopias, pancreatic accessory lobes and ducts, esophageal atresia, duodenal duplication or urogenital organ duplications. Furthermore, 19% of the 104 cases in the English literature had undergone malignant transformation, ten of which (56%) were gastric adenocarcinomas [12], four (22%) papillary adenocarcinomas [13], two (11%) GISTs [14] and two (11%) squamous cell carcinomas [15].
GDC may occur along the greater curvature (20%), followed by the posterior wall of the stomach (12%), the antrum (11%), along the lesser curvature (6%), the fundus (6%) and the pylorus (6%). Intrapancreatic location is extremely rare accounting for 3% of GDC and previously reported in only three adults. Τhe duplication cyst may or may not be entirely separated from the stomach, but they always share a common wall. In 28% of cases, there was direct communication between the cyst and the gastric lumen [12]. Symptomatology of GDC is varied ranging from no symptoms (24%) to epigastric pain (45%) [16], emesis (22%), nausea (21%) [17] and weight loss (13%). Other infrequent symptoms are nonspecific abdominal distention, melena [18], appetite loss, hematemesis [3], anemia [19], fever [20] and gastroesophageal reflux, depending on the location and characteristics of the cystic formation.
Intrapancreatic GDC, may erode into the pancreatic duct, eventually obstructing it leading to pancreatitis, while other symptoms described above may also occur [21,22], as described in Table 2. The reason for the symptomatology is yet not fully understood. Possible obstruction of the pancreatic duct by blood or blood clot may cause haemoductal pancreatitis. Furthermore, obstruction may occur due to mucoid secretions from the GDC, resulting to pancreatic juice stasis and an episode of acute pancreatitis. However, the fact that this symptomatology occurs in patients whose GDCs do not communicate with the pancreatic ductal system, like our case, or do not have an aberrant pancreatic duct, indicates different underlying mechanisms. Table 3 summarises GDC cases with aberrant pancreatic lobes, aberrant pancreatic ducts or cystic wall with pancreatic tissue (Tables 2 and 3).
| References | Age, gender | Symptoms | Location in the pancreas | Size (max diameter) cm |
|---|---|---|---|---|
| Kamei et al. [18] | 45, F | Anemia | Tail | 7 |
| Thomopoulos et al. [23] | 26, F | Epigastric pain, nausea, emesis, food intolerance, weight loss | Head | 8 |
| Remil et al. [24] | 48, M | Multiple episodes of pancreatitis complicated by pancreatic duct strictures | Head | 6 |
| Our Case | 18, M | Multiple episodes of acute pancreatitis | Body | 7 |
Table 2: Intrapancreatic GDC cases reported to date. GDC: Gastric Duplication Cyst, M: Male, F: Female
| References | Age, gender | Location | Size (max.diameter) cm | Symptoms | Aberrant pancreatic lobe |
|---|---|---|---|---|---|
| Bradbeer et al. [25] | 17, M | Juxtapancreas | 4,5 | Epigastric pain | No |
| Traverso et al. | 32, F | Antrum | n/a | Recurrent pancreatitis | Yes |
| Floras et al. | 30, M | n/a | 0,7 | n/a | No |
| Hoffman et al. [15] | 18, F | Greater Curvature | n/a | Epigastric pain, nausea | Yes |
| Bearzi et al. | 53, F | Antrum | n/a | Abdominal Distention | No |
| Johnstone et al. | 41, F | Juxtapancreas | n/a | Epigastric pain, emesis | No |
| Whiddon et al. | 24, F | n/a | n/a | n/a | Yes |
| Muraoka et al. [26] | 46, F | Greater Curvature | 4.5 | Epigastric pain, nausea, emesis | Yes |
| Shinde et al. [27] | 49, F | Antrum | 5 | Epigastric pain | Yes |
| Oeda et al. [28] | 38, F | Antrum | 4 | Recurrent pancreatitis | No |
| Anindita Sinha et al. | 30, F | Antrum | n/a | Epigastric pain, emesis, weight loss | No |
| Dal Mo Yang et al. [29] | 30, F | Pylorus | 6 | Epigastric pain | No |
| Jung Seop Eom et al. [30] | 28, M | Antrum | 0,6 | Dyspepsia | No |
| Jai P Singh et al. [5] | 28, F | Antrum and Pylorus | 2 and 1.3 | Epigastric pain, nausea, emesis | No |
| Kamei et al. [18] | 45, F | Intrapancreatic | 7 | Anemia | No |
| Kathleen K Christians et al. [31] | 43, M | Pyloric Antrum | n/a | Epigastric pain | Yes |
| Aysel Türkvatan et al. [9] | 29, F | Antrum | 5.3 | Epigastric and Back pain, emesis, nausea | Yes |
| Matthew P Doepker et al. [32] | 28, F | Antrum | 6 | Epigastric pain, nausea, emesis | No |
| Rousek M et al. [16] | 22, F | Antrum | 5.6 | Epigastric pain, weight loss, appetite loss | Yes |
| Yun Feng et al. [5] | 41, M | Gastric body | 25 and 18 | Epigastric discomfort and distention | Yes |
Table 3: Summary of GDC cases with aberrant pancreatic lobes, aberrant pancreatic ducts or cystic wall with pancreatic tissue. M: male, F: female, n/a: Non applicable
Due to the rarity of this condition, the prevalent symptoms and the anatomy of the area, preoperative differential diagnosis is difficult for most, if not all, physicians. Most commonly the differential diagnosis consists of acute or chronic pancreatitis, pancreatic pseudocyst, pancreatic cancer and GIST. In rare cases, lymphangioma, leiomyoma, gastric adenocarcinoma or other cystic formations, such as bronchogenic cysts or hydatid cysts, were considered. In our case the prevalent diagnosis was that of a pancreatic pseudocyst based on the predisposing factor of familiar hypertriglyceridemia, the biochemical analysis, the CT and MRI findings, and the prior history of recurrent episodes of acute pancreatitis from a young age. He had also reacted well to the stent placement one year before the excision.
There is no gold standard radiological diagnostic method. In the past, X-rays with barium meal was the most commonly used method [19], but currently CT with contrast medium, MRI, endoscopic retrograde cholangiopancreatography and MRCP are the most utilized means of morphological exploration for these malformations [30]. In the most recent past positron emission tomography/CT has also been used [33]. However Endoscopic Ultrasonography (EUS) may become important in the future, as it allows visualization of the GDC with the adjacent structures and potentiates the use of a fine needle aspiration [23]. In literature there also have been cases in the most recent past that PET/CT has been used. On the other hand, older cases show the prevalent use of X-rays with barium meals, a radiological method that seems to have lost its ground.
The optimal treatment modality for GDC, once diagnosed is complete resection. Other surgical options are excision of the common wall, bypass, and partial or total gastrectomy [22]. Malignant transformation of the GDC is still best managed by surgical resection. A conservative approach may be considered for patients with communicating GDCs and in whom both gastric lumens were patent. Marsupialization and cyst drainage have been discouraged as gastric contents in contact with the unprotected cystic mucosa may cause ulceration while the malignant potential of the GDC cannot be excluded [4].
Conclusion
Intrapancreatic GDCs are extremely rare developmental malformations and due to their low prevalence related literature is lacking. Because of their rarity and non-specific symptomatology, timely diagnosis is difficult. The best course of action is thorough imaging examination with contrast enhanced CT, MRI and MRCP to rule out possible developmental defects of the pancreas. Following imaging, complete removal of the cyst and the affected pancreatic section is the method of choice, as drainage has only a temporary therapeutic effect and does not protect from the possible development of malignancy within the GDC.
Author Contributions
Flamourakis K drafted the manuscript and reviewed the literature. Konstadoulakis M and Memos N were the patient’s surgeons, reviewed the literature and contributed to manuscript drafting; Tiniakos D, Bouklas D and Myoteri D. performed the pathology examination of the surgical specimen, contributed to the manuscript drafting and were responsible for the revision of the manuscript for important intellectual content; all authors issued final approval for the version to be submitted.
Refernces
- Passos ID, Chatzoulis G, Milias K, Tzoi E, Christoforakis C, et al. (2017) Gastric duplication cyst (gdc) associated with ectopic pancreas: Case report and review of the literature. Int J Surg Case Rep 31: 109-113
[Crossref], [Google Scholar], [Indexed]
- Ríos SS, Noia JL, Nallib IA, Leon AL, Perez-Quintela BV, et al. (2008) Adult gastric duplication cyst: diagnosis by Endoscopic Ultrasound Guided Fine-Needle Aspiration (EUS-FNA). Rev Esp Enferm Dig 100: 586-590.
[Crossref], [Google Scholar], [Indexed]
- Abdalkader M, Al Hassan S, Taha A, Nica I (2017) Complicated gastric duplication cyst in an adult patient: Uncommon presentation of an uncommon disease. J Radiol Case Rep 11: 16-23
[Crossref], [Google Scholar], [Indexed]
- Abdulla MAM, Al Saeed M, Alshaikh SA, Nabar UJ (2017) Adenocarcinoma arising from a gastric duplication cyst: A case report and literature review. Int Med Case Rep J 10: 367-372.
[Crossref], [Google Scholar], [Indexed]
- Singh JP, Rajdeo H, Bhuta K, Savino JA (2013) Gastric duplication cyst: two case reports and review of the literature. Case Rep Surg 2013:605059.
[Crossref], [Google Scholar], [Indexed]
- Fukumoto K, Suzuki S, Sakaguchi T, et al. (2008) Adenocarcinoma arising from gastric duplication: A case report with literature review. Clin J Gastroenterol. 2008;1(4):148–152.
[Crossref], [Google Scholar], [Indexed]
- Kuraoka K, Nakayama H, Kagawa T, et al. (2004) Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: A case report with literature review. J Clin Pathol 57:428-431
[Crossref], [Google Scholar], [Indexed]
- Bhatti ZS, Anderson MA, Wasnik AP (2016) Complete gastric duplication in an adult with associated anomalies. Clin Imag 40: 244-246.
[Crossref], [Google Scholar], [Indexed]
- Turkvatan A, Erden A, Turkoglu MA, Bostanci EB, Disibeyaz S, et al. (2015) An unusual cause of recurrent pancreatitis: A gastric duplication cyst with an accessory pancreatic lobe. Turk J Gastroenterol 25:199-202.
[Crossref], [Google Scholar]
- Samona S, Berri R (2015) A case report and review of the literature of adult gastric duplication cyst. Case Rep Surg 2015:1-3.
[Crossref], [Google Scholar], [Indexed]
- Kim DH, Kim JS, Nam ES, Shin HS (2000) Foregut duplication cyst of the stomach. Pathol Int 50:142-145.
[Crossref], [Google Scholar], [Indexed]
- Yamasaki A, Onishi H, Yamamoto H, Lenaga J, Nakafusa Y, et al. (2016) Asymptomatic adenocarcinoma arising from a gastric duplication cyst: A case report. IJSCR 25: 16-20,
[Crossref], [Google Scholar]
- Sethi S, Godhi S, Puri SK (2018) Papillary adenocarcinoma in a gastric duplication cyst. Indian J Surg Oncol 9: 979-82.
[Crossref], [Google Scholar], [Indexed]
- Fernandez DC, Machicado J, Davogustto G (2016) Gastrointestinal stromal tumor arising from a gastric duplication cyst. ACG Case Rep J 3:175-177.
[Crossref], [Google Scholar], [Indexed]
- Hoffman M, Sugerman HJ, Heuman D, Turner MA, Kisloff B, et al. (1987) Gastric duplication cyst communicating with aberrant pancreatic duct: A rare cause of recurrent acute pancreatitis. Surgery 101: 369-72. [Crossref],
[Google Scholar], [Indexed]
- Rousek M, Kachlik D, Nikov A, et al. (2018) Gastric duplication cyst communicating to accessory pancreatic lobe: A case report and review of the literature. World J Clin Cases. 2018;6(16):1182-1188
[Crossref], [Google Scholar], [Indexed]
- Barussaud ML, Meurette G, Cassagnau E, Dupasc B, Le Borgne J, et al. (2008) Mixed adenocarcinoma and squamous cell carcinoma arising in a gastric duplication cyst. Gastroenterol Clin Biol. 2008 Feb;32(2):188-91.
[Crossref], [Google Scholar], [Indexed]
- Kamei K, Yasuda T, Satoi S, Ishikawa H, Sakamoto H, et al. (2013) Intrapancreatic gastric duplication cyst mimicking pancreatic cystic tumor. Clin J Gastroenterol 6:156-9.
[Crossref], [Google Scholar], [Indexed]
- Spivak H, Pascal RR, Wood WC, Hunter JG (1997) Enteric duplication presenting as cystic tumors of the pancreas. Surgery 121: 597-600.
[Crossref], [Google Scholar], [Indexed]
- Lee CL, Daud CZ, Ismail RB (2014) Intrapancreatic gastric duplication cyst: A rare cause of chronic abdominal pain in childhood. J Clinical Ultrasound 42:42–4.
[Crossref], [Google Scholar], [Indexed]
- Takahara T, Torigoe T, Haga H et al. (1996) Gastric duplication cyst: Evaluation by en¬doscopic ultrasonography and magnetic resonance imaging. J Gastroenterol 31: 420-24
[Crossref], [Google Scholar], [Indexed]
- Thomopoulos T, Farin C, Navez B (2016) Total laparoscopic treatment of an adult gastric duplication cyst with intrapancreatic extension. Am J Case Rep 17: 352-6.
[Crossref], [Google Scholar], [Indexed]
- Simon R, Zoog E, Philips G, Dowden J (2017) Intrapancreatic enteric duplication cyst masquerading as groove pancreatitis. ACG Case Rep J 4: e123.
[Crossref], [Google Scholar], [Indexed]
- Bradbeer JW (1959) An unusual foregut anomaly: An extragastric pouch communicating with the pancreatic duct. Br J Surg 46: 603-5.
[Crossref], [Google Scholar], [Indexed]
- Muraoka A, Tsuruno M, Katsuno G, Sato N, Murata T, et al. (2002) A gastric duplication cyst with an aberrant pancreatic ductal system: Report of a case. Surg Today 32:531-5.
[Crossref], [Google Scholar], [Indexed]
- Shinde T, Lindner J, Silverman J, Agrawal R, Dhawan M, et al. (2009) Gastric-duplication cyst with an aberrant pancreatic-ductal system: an unusual cause of recurrent abdominal pain. GIE 69. 377-9.
[Crossref], [Google Scholar], [Indexed]
- Oeda S, Otsuka T, Akiyama T, Ario K, Masuda M, et al. (2010) Recurrent acute pancreatitis caused by a gastric duplication cyst communicating with an aberrant pancreatic duct. Intern Med 49: 1371-5.
[Crossref], [Google Scholar], [Indexed]
- Yang DM, Kim HC, Choi SI, et al. Sonographic diagnosis of gastric duplication cyst communicating with the gastric lumen. J Clin Ultrasound. 2011 Nov-Dec;39(9):550-2.
[Crossref], [Google Scholar], [Indexed]
- Eom JS, Kim GH, Song GA, Baek DH, Ryu KD, et al. (2011) Gastric Duplication Cyst Removed by Endoscopic Submucosal Dissection. Korean J Gastroenterol 58: 346-9.
[Crossref], [Google Scholar]
- Christians KK, Pappas S, Pilgrim C, Tasi S, Quebbeman E, et al. (2013) Duplicate pancreas meets gastric duplication cyst: A tale of two anomalies. Int J Surg Case Rep 4:735-9.
[Crossref], [Google Scholar], [Indexed]
- Doepker MP, Ahmad SA (2016) Gastric duplication cyst: A rare entity. J Surg Case Rep 2016: rjw073.
[Crossref], [Google Scholar], [Indexed]
- Tessely H, Montanier A, Chasse E (2018) Gastric duplication cyst with elevated CEA level: A case report. J Surg Case Rep 2018.
[Crossref], [Google Scholar], [Indexed]
- Woolfolk G, McClave S, Jones WF, Oukrop RB, Mark MD (1998) Use of endoscopic ultrasound to guide the diagnosis and endoscopic management of a large gastric dupli¬cation cyst. Gastrointest Endosc 47: 76-79. [Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences