10th World Congress on Rare Diseases and Orphan Drugs July 22-23, 2020 Barcelona, Spain
Ali Karami
Baqiyatallah University of Medical Sciences, Iran, E-mail: alikarami1@yahoo.com
Importance & Scope
There are more than 6000 Rare Diseases. Many rare diseases are diagnosed at the age of childhood, making diagnostic awareness and knowledge on treatment and care particularly important for pediatricians.
The Rare Diseases are so rare that rarity can lead to several problems including: difficulties in obtaining timely, accurate diagnoses; lack of experienced healthcare supervisors; useful, reliable and timely information may be difficult to retrieve; research activities are very less; developing new pharmaceuticals may not be economically feasible; treatments are sometimes very expensive; and in developing countries, the problems are compounded by other resource limitations.
Rare Diseases 2020 is emerging up with an exciting and high knowledge gaining conference program this year also which includes plenary lectures, symposia, workshops on a variety of topics, poster presentations and various programs for participants from throughout the world. We invite you to join us at the Rare Diseases and Orphan Drug Congress 2019 in Berlin where you will be sure to have a meaningful experience with scholars from around the world. The Rare Diseases Conference aims to extend the reach, impact and exchange of scholarly ideas through innovative technology, determined commitment and exceptional service. It aims to provide international collaboration and exchange of comprehensive and cuttingedge information on basic, translational, and applied clinical research on Rare Diseases and Orphan Drugs.
Market Research Growth on Rare Diseases and Orphan Drugs
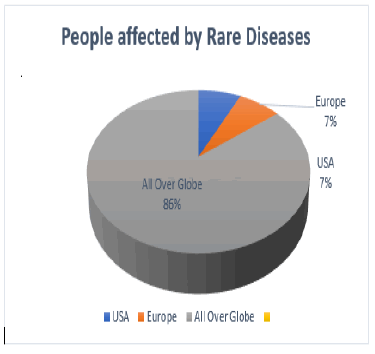
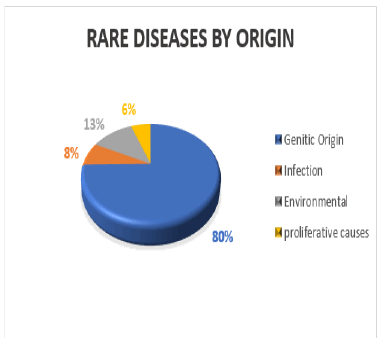
Nearly there are 7,000 different types of Rare Diseases and disorders it has estimated that 30 million people in the United States, 30 million in Europe and 350 million people over the globe suffer from Rare Diseases. Four by fifth of Rare Diseases are genetic in origin, among them affected individuals 50% of them will be children. The Rare Diseases are distributed in such a way that four fifth of the cases accounted by some 350 Rare Diseases. About only 5% of rare diseases are having approved drug treatment with only 326 new drugs being approved from the FDA and brought in to the market.
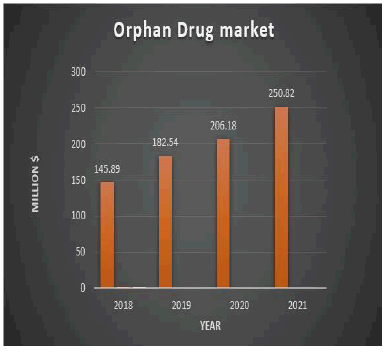
In 2018, Orphan Drug sales were of the order of 93 billion. Orphan Drugs represented 35% of the industry’s new drug approvals. The genetic diseases are subdivided by therapeutic area, which was leading the global market in the past will show similar traction in the coming next eight years. This segment is anticipated to be valued at US$ 56,241 by the end of 2025. According to Statistics of MRC, the Global Orphan Drug market is estimated at $145.89 million in 2018 and is expected to reach $265.63 million by 2022 growing at a CAGR of 10.5% from 2018 to 2022.
Major Associations Associated with Rare Diseases Research
• Alzheimer's disease Organizations
• Ann & Robert H. Lurie Children’s Hospital
• Birmingham children’s Hospital
• Boston Children’s Hospital
• Canadian Organization for Rare Diseases
• Chicago Rare Disease Foundation
• Children’s hospital of Pittsburgh
• Comer Children’s Hospital – University of Chicago
• Cystic Fibrosis Foundation
• European Union Committee of Experts on Rare Diseases
• EURORDIS Rare Diseases Europe
• EveryLife Foundation for Rare Diseases
• Global Genes Allies in Rare Diseases
• Guardian Hands Foundation
• Hospitals Associated with Rare Diseases Research
• IRDR Intractable & Rare Diseases Research
• Japan Patient Association
• Multiple Myeloma Research Foundation
• National Alliances for Rare Diseases
• National Institute of Health (NIH)
• National Organization for Rare Diseases
• NDC Medicine
• Organization for Rare Diseases India (ORDI)
• Orphan Europe
• Philippine Society for Orphan Disorders
• Rare Disease UK
• Rare Diseases or Syndromes and Clinical Societies
• Rare Diseases Foundation, Iran
• Rare Diseases Patient Association Funding
• Rare Diseases South Africa
• Rare Diseases Translational Research Collaboration
• Rare Disorders Society Singapore
• RARE Foundation Alliance
• Royal Society of Medicine
• Short Bowel Syndrome Foundation
• Students4RareDiseases
• The Asia-Pacific Alliance of Rare Disease Organisations
• The Boler-Parseghian Center for Rare & Neglected Diseases
• The Boler-Parseghian Center for Rare & Neglected Diseases
• The Children’s Hospital of Philadelphia
• The Every Life Foundation for Rare Diseases
• The Genetic and Rare Disorders Organization
• The Greek Alliance for Rare Diseases
• The Manton Center for Orphan Disease Research
• U.S. Food and Drug Administration
• UCLA Health
• US hospital for Rare Disease Research
Major Universities Associated with Rare Diseases Research
• Anahuac University North Campus, Mexico
• Australian National University, Australia
• Benha University, Egypt
• Birmingham City University, UK
• Center for Clinical Pharmacology, Belgium
• Center for Rare Neurological Diseases, USA
• Charles Darwin University Casoria Australia
• Columbia University Medical Center, United States
• Columbia University, USA
• Curtin University Bentley, Australia
• Dar Al Uloom University, Saudi Arabia
• Duke University, USA
• Emory University, USA
• GMEC, The Global Medical Excellence Cluster
• Guangzhou Medical University, China
• Harvard University, United States
• Harvard University, USA
• Imperial College London, United Kingdom
• Infection Control University
• Iqbal Chest Centre, Bangladesh
• John Hopkins University, USA
• Johns Hopkins University, United States
• Karolinska University, Sweden
• Kindai University, Japan
• King Saud Bin Abdulaziz University, Saudi Arabia
• King's College London, UK
• Kumamoto University, Japan
• Linnaeus University, Sweden
• Macquarie University, Australia
• Mayo Clinic College of Medicine, USA
• McGill university Montréal, Canada
• McMaster University, Canada
• Medi7 Bentleigh, Australia
• Murdoch University Murdoch, Australia
• National Institutes of Health, USA
• Newcastle University, Australia
• Northwestern University, Qatar
• Osaka University, Japan
• Oxford University, UK
• Philip Morris International R&D, Switzerland
• Pompeu Fabra University, Spain
• Queen Mary University, UK
• Radboud University Medical Center, Netherlands
• Rare Genomics Institute, USA
• Research Institute of Hospital del Mar, Spain
• Rizzoli Orthopedic Institute, Italy
• Samsung Medical Center, South Korea
• St George’s University of London, UK
• St. George Hospital, Australia
• Stanford University, USA
• Tasmanian Health Service, Australia
• The Chest & Heart Association of Bangladesh, Bangladesh
• The Fourth Hospital of Harbin Medical University, China
• The Jikei University School of Medicine, Japan
• The Third Affiliated Hospital of Guangzhou Medical University, China
• The University of Newcastle, Australia
• Tufts university, United States
• United Hospital, Bangladesh
• University College London, UK
• University of British Columbia, Canada
• University of Buffalo, United States
• University of California Los Angeles, United States
• University of California, USA
• University of Cambridge, USA
• University of Canberra Bruce, Australia
• University of Celiac Disease Center, USA
• University of Chicago Medicine, USA
• University of Colorado, USA
• University of Groningen, Netherlands
• University of Lincoln, UK
• University of London Imperial College of Science Technology and Medicine, UK
• University of Maastricht, Netherlands
• University of Maryland Medical Center, Australia
• University of Maryland Medical Center, United States
• University of Melbourne, Australia
• University of Minnesota, United States
• University of Newcastle, Australia
• University of Pennsylvania, USA
• University of Pittsburgh Study
• University of Pittsburgh, USA
• University of Queensland, Australia
• University of Tasmania, Australia
• University of Toronto, Canada
• University of Valencia, Spain
• University of Washington, USA
• University of Zurich, Switzerland
• University-of-the-sunshine-coast, Australia
• Weill Cornell Medical College, Qatar
• Wits University, South Africa
• Yale University School of Medicine, USA
• Yonsei University, South Korea
Major Companies Associated with Rare Diseases and Orphan Drugs
• 700thespians Limited, UK
• Abbott Laboratories, UK
• Actavis, USA
• Aegerion Pharmaceuticals, Japan
• Alexion Pharmaceuticals Inc, Switzerland
• Almirall, Spain
• Amgen, USA
• Amicus Therapeutics, USA
• Amphastar Pharmaceuticals, Inc
• Araim Pharmaceuticals
• Astellas Pharma US
• AstraZeneca, Switzerland
• AstraZeneca, UK
• Baxter International Deerfield
• Bayer HealthCare, Germany
• Bayer, Germany
• Beacon Pharmaceuticals, Bangladesh
• Biotie Therapies Corp, Finland
• Bioxyne Limited, Australia
• Boehringer Ingelheim, Germany
• Celgene, Switzerland
• Centrapharm Ltd, UK
• Chiesi Pharmaceutical, Italy
• Clalit Health Service, Israel
• Cohero Health, USA
• Daiichi Sankyo Europe, Germany
• Daiichi Sankyo, Japan
• Dohmen Life Science
• Elma Reseach, UK
• European Medicines Agency
• Focus Scientific Research Center (FSRC), phamax, India
• Forest Laboratories, USA
• Gecko Health, USA
• Generics (UK) Ltd, UK
• Genus Oncology Vernon Hills, USA
• Genzyme, USA
• Gilead sciences, USA
• GlaxoSmithKline, UK
• Global Data, UK
• Gsk, London
• Hormosan Pharma, Germany
• Hormosan Pharma, Germany
• Ikris Pharma Network Pvt Ltd, India
• Ikris Pharma Network Pvt Ltd, India
• Kissei Pharmaceutical Co., Ltd, Japan
• Kyowa Hakko Kirin Co. Ltd, Japan
• Lallemand Pharma, Switzerland
• Life Arc, UK
• Marathon Pharmaceuticals
• Merck & Co, USA
• Millennium Pharmaceuticals
• Napp Pharmaceuticals Ltd, UK
• Novartis, Switzerland
• NPS Pharmaceuticals
• Octapharma, USA
• Onyx Pharmaceuticals, USA
• Otsuka Holdings Co., Ltd, Japan
• Panmira Pharmaceuticals, LLC, USA
• Pearl Therapeutics, Inc
• Pfizer, USA
• PharmaMar, USA
• Prosensa, Netherlands
• PT Boehringer Ingelheim, Indonesia
• Queensland Respiratory Laboratory Pty. Ltd, Australia
• Ranbaxy Laboratories Limited
• Raptor Therapeutics
• Roche, Switzerland
• Samsung Medical Center, South Korea
• Sanofi, France
• Sarepta Therapeutics, USA
• Sigma-Tau Pharmaceuticals, Italy
• Sunovion Pharmaceuticals, USA
• Swedish Orphan Biovitrum AB, Sweden
• Takeda, Japan
• TriStem Corp Ltd, UK
• Vertex Pharmaceuticals, USA
• ViroPharma
• ViroPharma, USA
• Visionary Pharmaceuticals, Inc, USA
• Yungjin Pharm Ind. Co., Ltd, South Korea
Summary
Rare Diseases are that affects a small percentage of the population all over the world. An Orphan Disease is Rare Diseases with a lack of a market large enough to gain support and sources for discovering treatments for it, with advances to research in orphan diseases advantageous conditions to creating and selling such treatments. Rare diseases are genetic and are present throughout the entire life of individual suffering, even if symptoms do not immediately appear. Many Rare Diseases seems to appear early in life, and about 30% of children affecting with rare pediatric diseases will die before getting 5 year old. Rare Diseases are generally genetic and so they chronic. It is estimates that at least 80% of them have identified genetic origins. Other Rare Diseases are the result of infections and allergies or it may be due to degenerative and proliferative causes and discovering the well-advanced treatments in Rare Diseases and Orphan Drugs.
Orphan drugs 2020 Young Scientist Awards
Pharmaceutical Sciences Conference Committee is intended to honour prestigious award for talented Young researchers, scientists, Young Investigators, Post-Graduate students, Post-doctoral fellows, Trainees, Junior faculty in recognition of their outstanding contribution towards the conference theme. The Young Scientist Awards make every effort in providing a strong professional development opportunity for early career academicians by meeting experts to exchange and share their experiences on all aspects of Pharmaceutical Sciences.
Young Research’s Awards at Orphan drugs 2020 for the Nomination: Young Researcher Forum - Outstanding Masters/Ph.D./Post Doctorate thesis work Presentation, only 25 presentations acceptable at the Orphan drugs 2020 young research forum.
Guidelines for Young Researchers Forum
Benefits
• Young Scientist Award recongination certificate and memento to the winners.
• Our conferences provide best Platform for your research through oral presentations.
• Learn about career improvement with all the latest technologies by networking.
• Young Scientists will get appropriate and timely information by this Forum.
• Platform for collaboration among young researchers for better development.
• Provide an opportunity for research interaction and established senior investigators across the globe in the field.
• Share the ideas with both eminent researchers and mentors.
• It’s a great privilege for young researchers to learn about the research areas for expanding their research knowledge.
Eligibility
• Young Investigators, Post-Graduate students, Postdoctoral fellows, Trainees, Junior faculty with a minimum of 5 years of research experience
• Presentation must be into scientific sessions of the conference.
• Each Young Researcher / Young Scientist can submit only one paper (as first author or co-author).
• Age limit-Under 35yrs
• All submissions must be in English.
• Orphan drugs 2020 provides best platform to expand your network, where you can meet scientists, authorities and CROs from around the world. It’s your time to grab the opportunity to join Orphan drugs 2020 for promoting your research article and to facilitate prestigious award in all categories. In this fame, we look forward for your contribution and astonishing dedication to make our Orphan drugs 2020 more successful.
-
10th World Congress on Rare Diseases and Orphan Drugs
Barcelona, Spain
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
