AIDS Dementia Complex with Positive 14-3-3 Protein in Cerebrospinal Fluid: A Case Report and Literature Reviewa
Yunsen He1, Xiaohong Qin2, Min Feng4, Qinjiang Huang3, Mengjun Zhang2, Lili Guo1, Mingbin Bao1, Ye Tao1, Hongyuan Dai*3, and Bo Wu*1
1.Department of Neurosurgery, University of Electronic Science and Technology of China, Chengdu, China 2.Department of Neurosurgery, Wenjiang District People’s Hospital of Chengdu, Chengdu, China 3.Department of Psychiatry, Sichuan Provincial Center for Mental Health, Chengdu, China 4.Department of Geriatrics, municipal people's hospital, Luzhou, China
Published Date: 2023-10-30DOI10.36648/2380- 7245.9.5.95
Yunsen He, M.D.1*; Xiaohong Qin, M.D.2*; Mengjun Zhang, M.D.2; Lixin Liao M.D.2; Huping Chen, M.D.3; Qinjiang Huang, M.D.1; Lili Guo, Ph.D.1; Mingbin Bao, M.D.1; Qiang Zheng M.D.1; Hongyuan Dai, M.D.4; Bo Wu, M.D&Ph.D.1
1Department of Neurosurgery, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, PRC.
2Department of Psychiatry, Sichuan Provincial Center for Mental Health, Chengdu, Sichuan Province, PRC.
3Department of Hyperbaric Oxygen, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, PRC.
4Department of Neurology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, PRC.
- *Corresponding Author:
- Yunsen He
Department of Neurosurgery,
Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, School of Medicine,
University of Electronic Science and Technology of China, Chengdu, Sichuan Province,
PRC,
Tel: +86-028-87394131
Xiaohong Qin
Department of Psychiatry,
Sichuan Provincial Center for Mental Health, Chengdu, Sichuan Province,
PRC
Email: xhqin@dhu.edu.cn
Received date: October 08, 2023, Manuscript No. IPRDDT-23-17895; Editor assigned date: October 10, 2023, PreQC No. IPRDDT-23-17895 (PQ); Reviewed date: October 17, 2023, QC No. IPRDDT-23-17895; Revised date: October 23, 2023, Manuscript No. IPRDDT-23-17895 (R); Published date: October 30, 2023, DOI: 10.36648/2380-7245.9.5.95
Abstract
HIV-Associated Dementia (HAD) is a subcortical form of dementia characterized by memory deficits and psychomotor slowing. However, HAD can be confused with Creutzfeldt-Jakob disease (CJD), especially in AIDS patients. We report the case of a 54-year-old male patient presenting cognitive dysfunction and secondary behavioral changes after infection with HIV and suspicious prion. The patient was found to be infected with HIV during hospitalization and the Cerebrospinal Fluid (CSF) tested positive for 14-3-3 proteins; the electroencephalogram presented a borderline-abnormal periodic triphasic wave. Contrast-MRI revealed medium encephalatrophy and demyelination. Symptomatic treatment and adamantane were initially adopted for CJD, but the condition continued deteriorating. In contrast, various symptoms were alleviated after the first anti-HIV intervention. It is also the only surviving patient (over four years) with this prognosis. Therefore, the diagnosis was amended to HAD. When providing an etiological diagnosis of Rapidly Progressive Dementia (RPD), we should exclude as many causes as possible and perform an autopsy if necessary to reduce diagnostic bias. 14-3-3 protein should not be considered as the sole marker of CJD. Laboratory screening for infection indicators is required to improve diagnostic accuracy, especially in cases of AIDS with CJD. Finally, a diagnostic treatment may play a role when further examinations are unavailable.
Keywords
HIV-associated dementia; Cognitive dysfunction; Creutzfeld-Jakob disease; Rapidly progressive dementia
Introduction
HIV-Associated Dementia (HAD) is subcortical dementia characterized by memory deficits and psychomotor slowing, which occurs after the brain is infected with the Human Immunodeficiency Virus (HIV) [1,2]. Cognitive dysfunction is a common symptom in patients with AIDS and non-opportunistic infections caused by other viruses. On the other hand, the Creutzfeldt Jakob Disease (CJD) also known as Cortico-striatum- myeloid degenerative disease is characterized by mental disorders, dementia, parkinson-like manifestations, ataxia, myoclonus and muscle atrophy. CJD is a chronic and progressive disease caused by a rare infection with the prion protein (PrPSc) [3]. Simultaneously, the CSF 14-3-3 protein is an essential marker for diagnosing CJD. Here, a rare case of AIDS in a patient with a positive 14-3-3 protein is presented. Although similar cases have been reported, this case provides new information to the existing literature and is an important learning point for managing patients with rapidly progressive dementia due to its distinct diagnosis, treatment and efficacy [3-8].
Case Report
A 54-year-old male (Han ethnicity) was admitted to the neurology clinic of our institution. The patient had experienced six months of slurred speech, which had aggravated over the last three months. He had a memory disorder for over a year and mainly presented with progressive hypomnesis and paroxysmal anterograde amnesia. He also developed an unsteady gait. The initial diagnosis was "brain atrophy," with worsening of some symptoms after management at another institution without a case report. One year ago, the patient appeared inarticulate, with pain at the root of the tongue combined with malaise.
Additionally, dizziness and left ear tinnitus were occasionally noted but he did not present physical signs of dysphagia or choking. The patient rejected therapy until three months ago when the above conditions were aggravated and the patient became unable to take care of himself. During the first consultation in our clinic on July 13, 2018, the patient demonstrated advanced manifestations of unsteady gait with one reported fall, severe cognitive dysfunction, hypopsychosis, which gradually became silent and the amount of speech significantly decreased. Functionally, the patient was unemployed and lost self-care ability. Other past medical history was unremarkable. The patient had no history of exposure to toxic substances or family history of specific genetic diseases.
We performed a neurological physical examination after the patient visited the hospital for the first time. The patient was conscious and walked into a ward with a normal gait. Slurred speech, uncontrolled frowning and pursing were observed, and existing abnormalities on examination were consistent with cortical function deficits, including memory dysfunction, orientation, logical thinking and emotional expression. Meningeal stimulation did not reveal any abnormalities. Meanwhile, the pharyngeal reflex was reduced, muscle tension increased in the limbs, the counterattack sign was positive, the bilateral finger-to-nose (left side was obvious) and the heel-to-knee test demonstrated slight in coordination. Additionally, the Romberg sign was positive, but examination of the tongue deviation and other pathological signs demonstrated no abnormalities.
Assessments
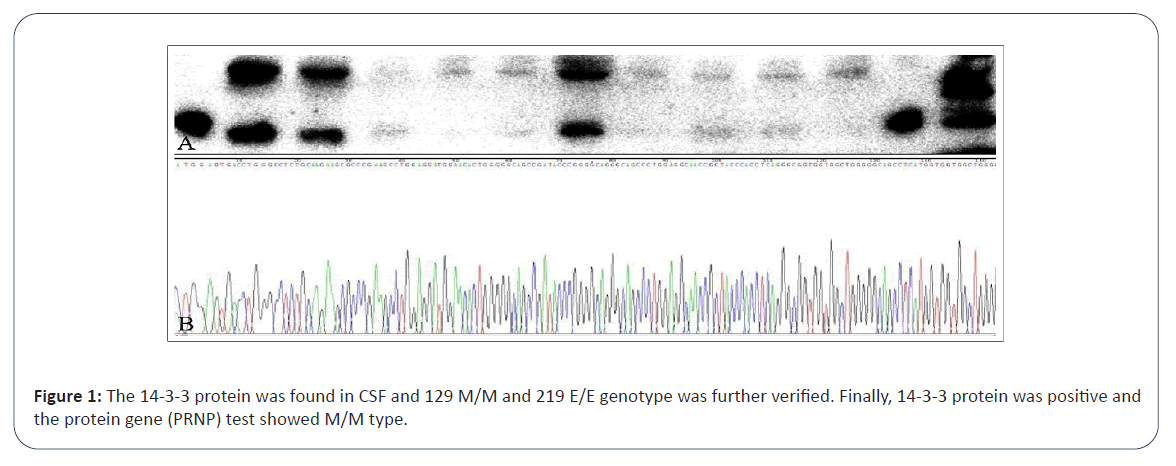
Laboratory tests: On admission to our institution, the family reported abnormality on the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) tests at another hospital; however, medical records were not provided. After hospitalization, HIV antibody screening test was positive and we carried out HIV antibody supplement test on the patient's blood (the final result is not back yet at that time). Laboratory tests revealed leukocytopenia, with a white blood cell count of 3.200 × 109/L and Lymphocyte (LYM) of 0.960 × 109/L. T cell subset components were detected, TH/TS ratio was 0.1 and T helper/ inducer (T4) cells accounted for 78/UL, while T suppressor/killer (T8) cells were 1218/UL. Additionally, the protein concentration in the CSF increased to 0.73 g/L. CSF cell count was within the normal range and other CSF analyses were performed for biochemical, routine culture (bacteria, fungi, Mycobacterium tuberculosis and Cryptococcus) characterization and the antibody test related to autoimmune and paraneoplastic encephalitis was negative. No abnormalities were found in liver and kidney function, anti-thyroid peroxidase antibody, anti-thyroglobulin antibody, ceruloplasmin, antinuclear antibody, anti-neutrophil cytoplasmic antibody, folic acid, or vitamin B12. The 14–3–3 protein was detected in the CSF, and the 129 M/M and 219 E/E genotypes were further verified (Figure 1). Electroencephalogram (EEG) revealed a borderline abnormality of the periodic triphasic wave rather than a typical disorder.
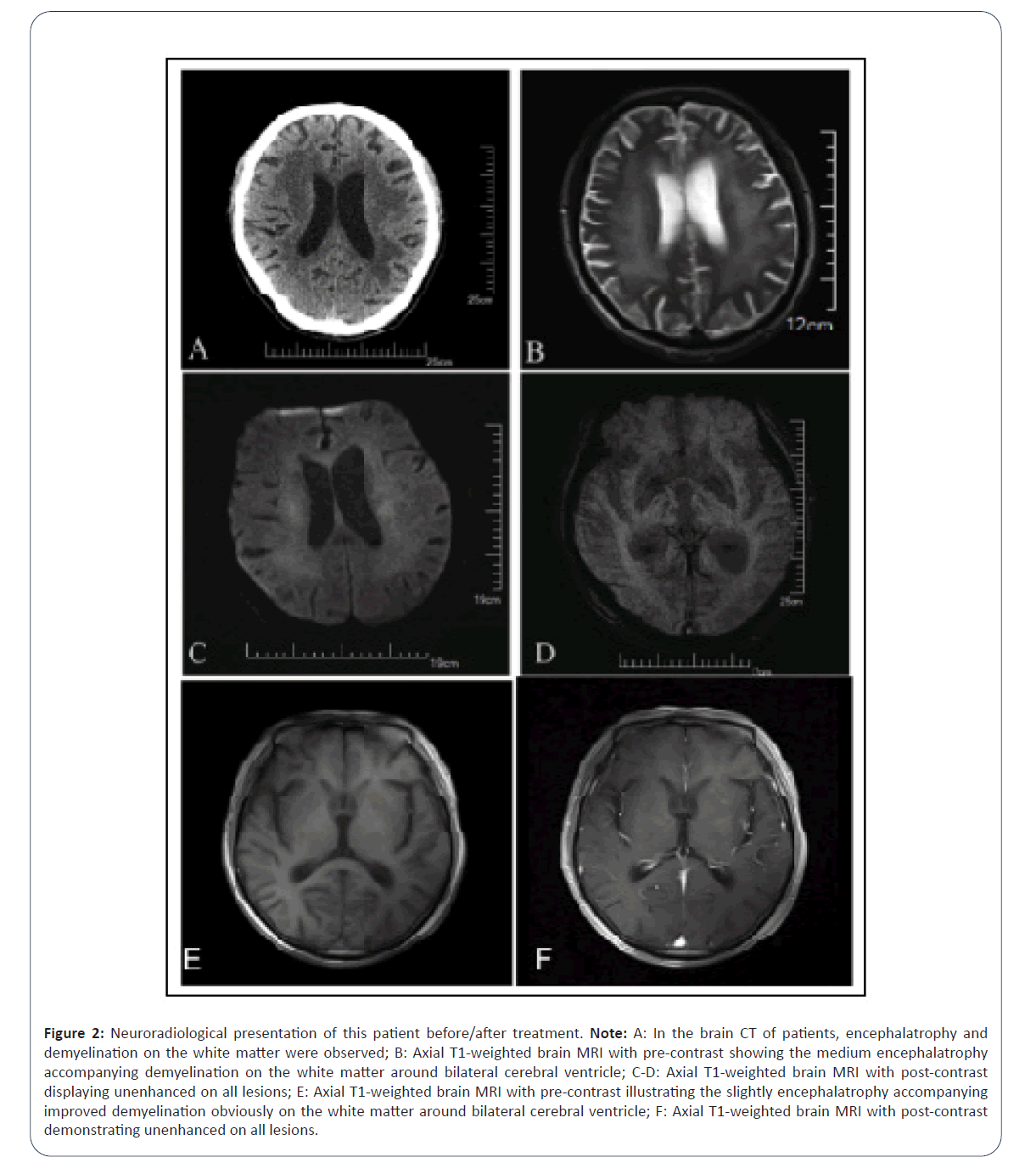
Neuroradiological results: Craniocerebral Computed Tomography (c-CT) revealed encephalatrophy and demyelination abnormalities in the white matter, given the multiple punctate low-density white matter exhibited in the bilateral basal ganglia with non-enhancement in all lesions and was initially diagnosed with lacunar infarction (Figure 2A-2F).
Figure 2: Neuroradiological presentation of this patient before/after treatment. Note: A: In the brain CT of patients, encephalatrophy and demyelination on the white matter were observed; B: Axial T1-weighted brain MRI with pre-contrast showing the medium encephalatrophy accompanying demyelination on the white matter around bilateral cerebral ventricle; C-D: Axial T1-weighted brain MRI with post-contrast displaying unenhanced on all lesions; E: Axial T1-weighted brain MRI with pre-contrast illustrating the slightly encephalatrophy accompanying improved demyelination obviously on the white matter around bilateral cerebral ventricle; F: Axial T1-weighted brain MRI with post-contrast demonstrating unenhanced on all lesions.
Diagnosis and management
The patient's condition worsened while waiting for the definitive diagnosis of AIDS and we treated the patient with symptomatic treatment and amantadine (amantadine hydrochloride tablets, USP) for CJD, which was the initial diagnosis considered. However, the condition of the patient continued to deteriorate. At that time, HIV antibody supplement tests were positive.
The patient was then transferred to a local epidemic prevention institution for further treatment. The details of the therapeutic regimen with anti-HIV medication were obtained from the local institution over four years and included the following: Efavirenz (600 mg QD), tenofovir disoproxil (300 mg QD), lamivudine (100 mg QD) and compound sulfamethoxazole tablets (480 mg BID). Subsequently, the various symptoms rapidly improved after anti- HIV therapy during hospitalization.
Follow-up
After four years, we performed an exhaustive re-examination during outpatient follow-up. The patient's consciousness was transparent with articulate speech; the recent memory and emotional expression ability were reduced but only slightly lower than normal; and the orientation and logical thinking functions were unremarkable. The limb muscle tension slightly increased and the muscle strength was normal. The neurological signs and other symptoms were normal. The neuroradiological reexamination of the c-MRI revealed that the slight encephalatrophy imaging accompanying obvious demyelination in the white matter around the bilateral cerebral ventricle had improved than previous imaging. Meanwhile, several punctate low-density lesions were detected at the bilateral basal ganglia with a nonenhancement.
Finally, the scales or other examinations before and after the intervention are listed in Table 1.
| Test items | Before | After |
|---|---|---|
| MoCA | 16 | 25 |
| ADL | 25 | 70 |
| Muscle strength | V | V |
| Hypertonia | (+ï¼? | Improvement |
| Pathological reflex | ï¼?-ï¼? | ï¼?-ï¼? |
| Neuroradiology | ï¼?+ï¼? | Improvement |
| Note: MoCA: Montreal Cognitive Assessment; ADL: Activities of Daily Living. | ||
Table 1: Comparison of conditions between before and after treatment with anti-HIV.
Amended diagnosis
Considering the atypical MRI findings, EEG abnormalities and the remarkable efficacy of anti-HIV treatment, we finally considered the diagnosis of HAD even when CSF 14-3-3 protein was positive.
Ethics
The patient gave his written informed consent for his laboratory results and publication of the article. The study protocol was approved by the Institutional Review Board of University of Electronic Science and Technology of China on human research.
Results and Discussion
CJD is a degenerative central nervous system disease caused by prion proteins, mainly manifested as progressive dementia, myoclonus, cerebellar dysfunction and a kinetic mutism [9]. The average survival from onset to death is only a few months [4-8]. According to its etiology, CJD is mainly divided into four types: Sporadic (accounting for approximately 85%), hereditary/family (5%-15%), iatrogenic and variant (0%-10%) [3]. s-CJD is diagnosed based on rapidly progressing cognitive impairment and must be confirmed by neuropathology or immunochemical or biochemical evidence [8]. Possible s-CJD requires not only symptomological evidence but also auxiliary examination, such as EEG with periodic discharge, DWI with ribbon sign, 14-3-3 protein positivity in CSF and RT-QuIC positive [10].
Reviewed the literature on s-CJD between 1995 and 2011 and screened out thirty-eight articles with 1849 s-CJD suspects with detection of the 14-3-3 protein [11]. The results depicted that 14-3-3 protein has diagnostic value for s-CJD (sensitivity, 92%; specificity, 80%). In addition, the sensitivity and specificity of prions detected by Real Time Quaking-Induced Conversion (RTQuIC) can be increased to 96% and 100% [12]. MRI sensitivity is 80% in CJD some studies put the sensitivity as high as 92% to 98% [13-19]. At the same time, its specificity is 74%-98% [13,15].
Periodic Sharp-Wave Complexes (PSWCs) with a frequency of 1 Hz are considered an EEG pattern typical of CJD and have revealed a sensitivity of 64% and a specificity of 91% [20]. Molecular subtypes of sporadic CJD are defined by codon 129 polymorphisms (M and V) and PrPSc glycotype (1 and 2), resulting in different subtypes (e.g., MM1 and MV1) [9]. A single somatic mutation of the PRNP gene located at M129V and E219K plays an essential role in the pathogenesis of CJD [21]. This gene frequency varies with race; the Han population had the highest risk of carrying 129 M/M genotype, with an earlier onset age. Typical PSWCs occur in late disease stages and are less frequent in MV2, VV2 and MM2 cases [10].
In such cases, the diagnosis of probable CJD should meet the criteria for symptomatology, ancillary tests and exclusion.
Symptomatology
First rapidly progressive cognitive impairment. A) Myoclonus; B) Visual or cerebellar disturbance; C) Pyramidal or extrapyramidal signs; D) Akinetic mutism.
Auxiliary: A) Typical EEG; B) Typical brain MRI; C) Positive CSF 14-3-3. Simultaneously, other possible diseases must also be excluded. Possible diagnostic criteria for CJD must meet the first and second symptoms (at least two), a positive criterion on a combined auxiliary test. Of course, other possible diseases must be ruled out to be diagnosed as probable CJD. A probable diagnosis of CJD is sufficient, in addition to meeting the criteria for the first and second symptoms (at least two), with a duration of less than two years. The protein gene (PRNP) test demonstrated M/M type, which increased the suspicion of CJD. Although there is a strong possibility that HAD was considered in this patient, probable CJD should still be considered during hospitalization in our hospital.
HAD is a common neurological complication after HIV infection and is mainly associated with memory loss, motor dysfunction, mental insufficiency, difficulty performing complex tasks and behavioral irregularities, such as apathy and abnormal responses [1,22].
Most patient’s present only short-term memory disorders in the early stage of AIDS; however, as the disease progresses, HIVrelated chronic inflammation and immune activation may involve multiple brain regions, leading to memory, cognitive, language expression and comprehension dysfunction. With the widespread application of Highly Active Anti-Retroviral Therapy (HAART), the survival of patients with HIV has been significantly prolonged, but the incidence of moderate neurocognitive impairment remained higher. The possible reason is that most anti-HIV drugs cannot efficiently cross the blood-brain barrier into the Central Nervous System (CNS), resulting in low blood drug concentration in the CNS. Combined with the environmental impact of the CNS, HIV is prone to mutation, and chronic accumulation of neurotoxicity leads to moderate neurocognitive dysfunction [22].
In this case, the diagnosis was considered infectious dementia combined with the medical history of the patient and auxiliary examination. The prime suspect was HIV, based on the following:
• Both HIV antibody screening test and HIV antibody supplement tests were positive.
• The apparent symptoms, including memory disorders, slowed mental processing and behavior disorder, were the primary symptoms of HAD and the ability to perform activities of daily living was significantly decreased.
• Regarding neuroradiology, CT and c-MRI revealed brain atrophy, demyelination and white matter changes with a non-enhancing effect.
Consequently, the information above was consistent with HIV infection.
Four years after the anti-HIV treatment, we noted improved cognitive function and self-care abilities. However, the memory remained worse than before; therefore, the prion may also play a role in RPD of the patient and we speculate that HIV and CJD are not entirely coincidental as previously suggested [5,7].
Patients with HIV and prion co-infection are very rare and to the best of our knowledge, only five cases have been diagnosed to date and three others are highly suspected, including our patient (Table 2).
| Author | Pt | Sex | Age | Race/Region | Symptoms | Examination | Diagnosis | Mana-gement | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| M-Alain et al. [5] | 2016 | Male | 66 | USA | Conceptual apraxia, apathy, memory impairment, and gait disturbance, ataxia with gait disturbance, chronic peripheral neuropathy | CSF: 14-3-3 (+); T-Tau (+); RT-Qu IC (+); MRI: Signal abnormalities in the bilateral caudate, putamen and thalami, as well as gyriform cortical; EEG: (-); PRNP: N/A; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (3 months) |
| Eimer et al. [8] | 2018 | Male | 59 | Caucasian | Mildly disoriented being insecure about the situation and location | CSF: 14-3-3 (+); MRI: Signal abnormalities in the caudate nuclei, frontal cortex and parietal cortex bilaterally; EEG: Periodic triphasic spike and wave complexes; PRNP: M129V; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (2 months) |
| Abu-Rumeileh et al. [4] | 2018 | Male | 62 | Italy | Drowsy, with reduced verbal fluency, miotic reagent pupils and a mask face. Axial and limb plastic hypertonia and dystonia of both hands | CSF: 14-3-3 (+); MRI: cortical atrophy and multiple white matter lesions. EEG: Pseudo-periodic slow spike discharges; PRNP: N/A; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (4 months) |
| de Carvalho Neto et al. [7] | 2019 | Male | 52 | Caucasian | Progressive imbalance, motor and cognitive deterioration and hypersomnia | CSF: 14-3-3 (+); MRI: cortical gyri_× 005 f form restriction on both hemispheres; EEG: Triphasic PSWC; PRNP: N/A; Autopsy: N/A | Probable sporadic CJD | Palliative care | Passed away (Greater than 2 months) |
| van de Ven et al. [22] | 2019 | Male | 63 | Black Zimbabwean | Progressive difficulties with decision-making, obsessive compulsive disorder and visual hallucinations. | CSF: 14-3-3 (weakly(+)); MRI: Bilateral abnormal signal within the posterolateral thalami compatible with pulvinar sign; EEG: Diffuse excess of slow activity; PRNP: M129V; Autopsy: CJD | Variant CJD | Palliative care | Passed away (10 months) |
| Dahy et al. [6] | 2021 | Male | 52 | Brazil | Global cerebellar syndrome, bilateral Babinski, 4-limb paratonia and release of face axial reflexes. The memory, attention and executive function deficits. | CSF: 14-3-3 (+); MRI: bilateral hyper intensity of images in caudal nuclei; EEG: (-); PRNP: M129V; Autopsy: N/A | Probable sporadic CJD | N/A | Passed away (13 months) |
| Dahy et al. [6] | 2021 | Male | 61 | Brazil | Asthenia, lack of appetite, difficulty sleeping and occasional memory lapses, uncoordinated steps, visual delusions | CSF: 14-3-3 (+); MRI: bilateral cortical ribboning in the cerebral cortex; PRNP: N/A; EEG: N/A; Autopsy: N/A | Probable sporadic CJD | N/A | Passed away (5 months) |
| Current report | 2022 | Male | 54 | Han/China | Progressive hypomnesis, paroxysmal anterograde amnesia, unsteady gait. | CSF: 14-3-3 (weakly(+)); MRI: Bilateral abnormal signal within the posterolateral thalami compatible; EEG: Borderline abnormality of the periodic triphasic wave; PRNP: 129 M/M; Autopsy: N/A | Probable ADC | Anti-HIV | Improved and following-up |
Table 2: The deference between previous case report about rapidly progressive dementia with HIV and current report.
Unlike previously reported cases, our patient demonstrated sustained improvement with anti-HIV therapy and was the only surviving patient. Our report provides a completely different reference for managing such cases.
The first patient was published by Babi et al. in 2016 [5]. This patient had well-controlled chronic AIDS, while the old man died three months after finding positive 14-3-3 protein in the CSF, and a diagnosis of sporadic CJD (s-CJD) was made histopathologically by autopsy. Subsequently reported cases, all patients died in 2-13 months [4-8,23]. In three of these cases, the diagnosis of sCJD was also confirmed by autopsy and Variant CJD in one case [4,8,23]. In the remaining two cases, the autopsy was unavailable, but CJD was highly suspected [6,7]. Most of these authors agree that there is no direct evidence of an association between HIV infection and prions, but further linkage is needed concluded that RT-QuIC should be used as a specific screen for progressive dementia, while Dahy et al. believe that screening for s-CJD is mandatory for young patients with dementia in people living with HIV [5-7,9,23].
In our case, the patient's symptoms improved after anti-AIDS treatment and was less likely to be diagnosed with CJD (Table 1). Although seven patients identified in previous case reports had a similar condition to the current report and presented with AIDS and 14-3-3 protein positivity, they were finally confirmed as having CJD by autopsy (Table 2).
In patients without routine HIV screening tests, RPD and positive 14-3-3 protein in CSF can easily be misdiagnosed as CJD [22-25]. Neurologists should do as much as possible to determine the cause of RPD when making the diagnosis. Positive 14-3-3 protein expression is of great value in CJD diagnosis, but it also has some limitations and presents interference. The CSF14-3-3 protein recheck or RT-QuIC test should be considered to improve diagnostic accuracy when the supplementary examination is unavailable for this rare case.
Conclusion
HAD and CJD are easily misdiagnosed. When providing an etiological diagnosis of RPD, we should exclude as many causes as possible and perform an autopsy if necessary to reduce diagnostic bias, while 14-3-3 protein should not be considered the only marker for CJD. Laboratory screening for infection indicators is required to improve diagnostic accuracy, especially in cases of AIDS with CJD. Finally, a diagnostic treatment may play a role when further examinations are unavailable.
Limitations
In this case, although HAD diagnosis was more likely based on all evidence, the diagnosis could not be confirmed by pathological examination because the patient remained healthy and alive.
In this case, we established that HAD played a major role in the patient's impaired consciousness and behavior. We are still unable to clarify whether prion infection is also involved in this pathological process; however, we did not perform RT-QuIC or neuropathologic tests on this patient to obtain more supportive evidence.
Disclosure of Funding Statements
There is no funding supporting the production of this manuscript.
Conflicts of Interest and Disclosure
None of the authors have any conflict of interest to be declared.
Ethic Approval and Patient Consent
Institutional Review Board (IRB) permission obtained for the study. And the patient has provided informed consent for the publication of the case.
Authorship Contribution Statement
Yunsen He and Xiaohong Qin contributed equally to this work and should be regarded as joint first authors. All authors made substantial contributions to the study design, data collection, methodology, software, original draft, critical revision, and final approval of this manuscript.
References
- McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. The Lancet Neurol 4: 543-555.
[Corssref], [Google Scholar]
- Navia BA, Jorday BD, Prince RW, Bradford A (1986) The aids dementia complex: I. Clinical features. Ann Neurol 19: 517-524.
[Corssref], [Google Scholar]
- Sitammagari KK, Masood W (2018) Creutzfeldt Jakob Disease. StatPearls Publishing, Florida, USA.
- Abu-Rumeileh S, Baiardi S, D'Angelo R, Dentale N, Fasulo G, et al. (2018) Clinical Reasoning: Rapidly progressive dementia in a patient with HIV after an exotic journey. Neurology 91:1360-1364.
[Corssref], [Google Scholar], [Indexed]
- Babi A, Kraft BD, Sengupta S, Peterson H, Orgel R, et al. (2016) Related or not? Development of spontaneous Creutzfeldt–Jakob disease in a patient with chronic, well-controlled HIV: A case report and review of the literature. SAGE Open Med Case Rep 13: 1-5.
[Corssref], [Google Scholar], [Indexed]
- Dahy FE, Novaes CTG, Bandeira GA, Ramin LF, Smid J, et al. (2021) Sporadic Creutzfeldt-Jakob disease in two clinically and virologically controlled Brazilian HIV patients who progressed rapidly to dementia: Case reports and literature review. Rev Inst Med Trop Sao Paulo 63: 23.
[Corssref], [Google Scholar], [Indexed]
- Neto EGC, Gomes MF, de Oliveira M, Guete MIN, Santos IP, et al. (2019) The worst is yet to come: Probable sporadic Creutzfeldt-Jakob disease in a well-controlled HIV patient. Prion 13:156-159.
[Corssref], [Google Scholar], [Indexed]
- Eimer J, Vesterbacka J, Savitcheva I, Press R, Roshanisefat H, et al. (2018) Nonopportunistic infection leading to rapidly progressive dementia in a patient with HIV/AIDS: A case report. Medicine (Baltimore) 97: 162.
[Corssref], [Google Scholar], [Indexed]
- Geschwind MD (2016) Rapidly progressive dementia. Continuum 22:510-537.
[Corssref], [Google Scholar], [Indexed]
- Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, et al. (2021) Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol 20: 235-246.
[Corssref], [Google Scholar], [Indexed]
- Muayqil T, Gronseth G, Camicioli R (2012) Evidence-based guideline: Diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 79: 1499-1506.
[Corssref], [Google Scholar], [Indexed]
- Lattanzio F, Abu-Rumeileh S, Franceschini A, Kai H, Amore G, et al. (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: Diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133: 559-578.
[Corssref], [Google Scholar], [Indexed]
- Hermann P, Laux M, Glatzel M, Matschke J, Knipper T, et al. (2018) Validation and utilisation of amended diagnostic criteria in Creutzfeldt-Jakob disease surveillance. Neurology 91: 331–338.
[Corssref], [Google Scholar], [Indexed]
- Franceschini A, Baiardi S, Hughson AG, McKenzie N, Moda F, et al. (2017) High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep 7: 10655.
[Corssref], [Google Scholar], [Indexed]
- Abu-Rumeileh S, Baiardi S, Polischi B, Mammana A, Franceschini A, et al. (2019) Diagnostic value of surrogate CSF biomarkers for Creutzfeldt-Jakob disease in the era of RT-QuIC. J Neurol 266: 3136-3143.
[Corssref], [Google Scholar], [Indexed]
- Fiorini M, Iselle G, Perra D, Bongianni M, Capaldi S, et al. (2020) High diagnostic accuracy of RT-QuIC assay in a prospective study of patients with suspected sCJD. Int J Mol Sci 21: 880.
[Corssref], [Google Scholar], [Indexed]
- Rudge P, Hyare H, Green A, Collinge J, Mead S (2018) Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry 89: 461–66.
[Corssref], [Google Scholar], [Indexed]
- Forner SA, Takada LT, Bettcher BM, Lobach IV, Tartaglia MC, et al. (2015) Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt Jakob disease. Neurol Clin Pract 5: 116–125.
[Corssref], [Google Scholar], [Indexed]
- Bizzi A, Pascuzzo R, Blevins J, Grisoli M, Lodi R, et al. (2020) Evaluation of a new criterion for detecting prion disease with diffusion magnetic resonance imaging. JAMA Neurol 77: 1141–1149.
[Corssref], [Google Scholar], [Indexed]
- Steinhoff BJ, Zerr I, Glatting M, Schulz-Schaeffer W, Poser S, et al. (2004) Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol 56: 702–08.
[Corssref], [Google Scholar]
- SY (2005) Polymorphisms of the PRNP gene in Chinese populations and the pathogenic mechanism research of a novel insertion mutant of PrP. Wuhan University.
- Lifeng RW (2015) Progress in neurotoxicity and its mechanism of human immunodeficiency virus. Neural Iniury and Functional Reconstruction 10: 432.
- Ven NS, Vera J, Jones JR, Vundavalli S, Ridha BH, et al. (2019) Sporadic CJD in association with HIV. J Neurol 266: 253-257.
[Corssref], [Google Scholar], [Indexed]
- Müller WE, Laplanche JL, Ushijima H, Schröder HC (2000) Novel approaches in diagnosis and therapy of Creutzfeldt–Jakob disease. Mech Ageing Dev 116: 193-218.
[Corssref], [Google Scholar], [Indexed]
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, et al. (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: 2659-2668.
[Corssref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences