Previously Undiagnosed Tuberous Sclerosis Complex in a Newborn: A Case Report
Yesim Coskun, Selcuk Gurel, Gulendam Kocak, Volkan Hazar, Ipek Akman and Ozge Yabas Kiziloglu
DOI10.21767/2380-7245.100165
Yesim Coskun1*, Selcuk Gurel1, Gulendam Kocak1, Volkan Hazar1, Ipek Akman1 and Ozge Yabas Kiziloglu2
1Department of Pediatrics, Goztepe Medicalpark Hospital, Bahcesehir University School of Medicine, Istanbul, Turkey
2Department of Ophtalmotology, Goztepe Medicalpark Hospital, Bahcesehir University School of Medicine, Istanbul, Turkey
- *Corresponding Author:
- Yesim Coskun
Department of Pediatrics
Goztepe Medicalpark Hospital
Bahcesehir University School of Medicine
E5 uzeri 23 Nisan sokak No: 1734732 Merdivenkoy/Goztepe
Istanbul, Turkey
Tel: +90 444 2 864
E-mail: coskunyesim@yahoo.com
Received Date: July 18, 2017; Accepted Date: October 13, 2017; Published Date: October 23, 2017
Citation: Coskun Y, Gurel S, Kocak G, Hazar V, Akman I, et al. (2017) Previously Undiagnosed Tuberous Sclerosis Complex in a Newborn: A Case Report. J Rare Disord Diagn Ther. 3:12. DOI: 10.21767/2380-7245.100165
Abstract
The early diagnosis of tuberous sclerosis complex (TSC), especially during the neonatal period is rare. If there is a family history, foetal cardiac masses diagnosed by foetal echocardiography, may suggest TSC. After the delivery, in the neonatal period, TSC should be rule out. We report a case who had TSC and diagnosed early in the neonatal period. We would like to describe the clinical features of TSC and emphasize the importance of early diagnosis.
Keywords
Antenatal foetal echocardiography; Cardiac rhabdomyoma; Newborn
Introduction
Tuberous sclerosis complex (TSC) is a multisystem tumor suppressor gene syndrome inherited autosomal dominantly, also known as a neurocutaneous disorder. It is characterized by benign tumors commonly of the heart, skin, brain, kidney and eye [1,2]. The incidence of TSC is about 1 in 6.000 at live birth [3]. Although the diagnosis of TSC may be difficult because of the absence of pathognomonic signs and symptoms, the diagnostic criteria defined by the International Tuberous Sclerosis Consensus Conference in 2012 are used for the diagnosis [4].
In this report, we present a newborn with biventricular massive myocardial masses who is diagnosed as TSC and discuss the clinical findings of TSC.
Case Report
The male baby was born full-term via a Caesarean section as the second child of a 24-year-old mother. The mother had no history of radiation exposure, drug ingestion, alcohol use, or smoking during pregnancy, and there was no consanguinity.
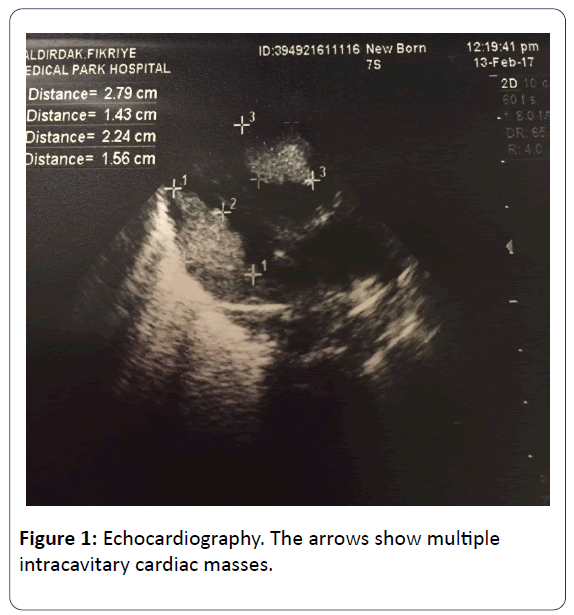
The family history revealed that his mother has TSC which was diagnosed when she was 10 years old. After birth, the infant was reffered to our NICU for dyspnea and tachypnea. At presentation, his body temperature was 36.9°C, blood pressure of 88/45 mmHg, heart rate of 135 beats per minute and the respiratory rate of 70 breaths per minute. His breath sounds were equal, he had grunting, nasal flaring, tachypnea, subcostal retraction and central cyanosis. The right lower extremity of the infant was edematous and swollen. Blood tests revealed elevated level of C-reactive protein (29.9 mg/L). Blood, endotracheal, and urine cultures were negative. Chest x-ray revealed pulmonary infiltration. The initial diagnosis was congenital pneumonia. A few hours later, intubation was performed and intratracheal surfactant was administered. The patient was on mechanical ventilation in the synchronized intermittent mandatory ventilation (SIMV) mode. On day 3, echocardiography (ECHO) showed multiple (more than 5) echogenic masses arising from both the left and the right ventricle which were not causing dynamic left and right ventricular outflow tract obstruction. Systolic and diastolic functions were in normal limits. Those echogenic lessions were reported as typical of rhabdomyomas (Figure 1). After the ECHO findings, the newborn was reffered to ophthalmologists for the presence of retinal pathologies.
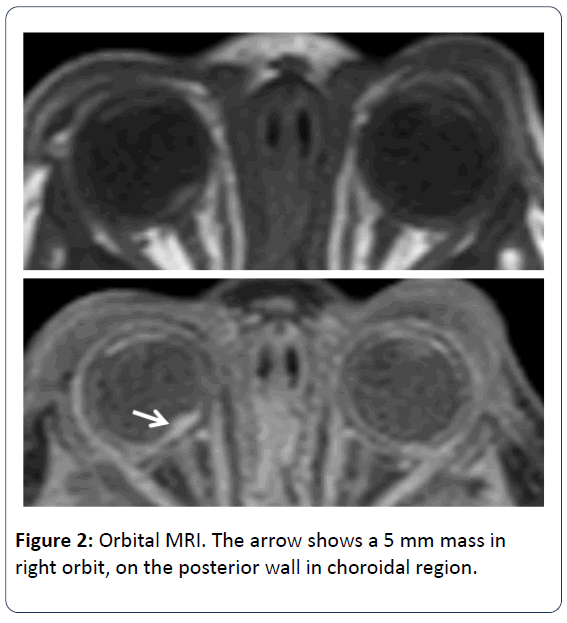
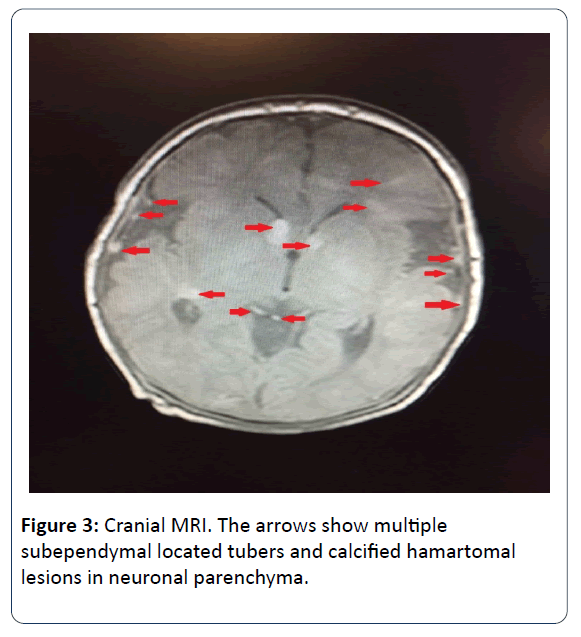
Ophtalmologic consultation revealed a yellow colored and lobulated structure on the right optic nerve as an asrocytic hamartoma. Ocular magnetic resonance imaging (MRI) revealed anterior palpebral soft tissue edema and a 5 mm mass was observed (in the right orbit, on the posterior wall in choroidal region) (Figure 2). Cranial MRI showed multiple subependymal located tubers and calcified hamartomal lesions in neuronal parenchyma (Figure 3). Right thigh MRI was obtained to evaluate the swelling of the extremity which showed multiple heterogenous signals and soft tissue edematous lesions. The patient was extubated on 7th day and followed on NCPAP, soon weaned to hood on 9th day. He administered antibiotics for 10 days for congenital pneumonia.
On the 22nd day of his admission, he was discharged from the hospital for follow up. During the follow up when he was 3 months old, he had strabismus like eye movements which were considered as seizure. EEG was performed. Parieto-central epileptic activity was detected and phenobarbital treatment was started. During the follow up of the ophtalmologic examination, the hamartoma didn’t regress. The cardiologic evaluation was performed monthly. There was no regression in the size and number of the cardiac masses. When he was 5 months old, insurance procedures were completed and everolimus treatment was started. After two months of therapy the patient was evaluated by ECHO. We obtained significantly reduction in the size of all cardiac masses.
Discussion
TSC is an autosomal dominant inherited disease with nearly 60-70% of cases are related with new mutations. Molecular studies showed that 75-90% of patients have mutations in TSC1 or TSC2 genes. TSC1 gene encodes hamartin and TSC2 gene encodes tuberin which regulate cellular growth and differentiation. Pathologies of these genes result in hamartoma formation during organogenesis [5,6]. Diagnostic criteria defined by the Tuberous Sclerosis Complex Consensus Conference in 2012 is used for the diagnosis of TSC. There are 2 diagnostic criteria; genetic and clinical. The identification of either a TSC1 or TSC2 pathogenic mutation in DNA from normal tissue is sufficient to make a definite diagnosis of TSC. However, 10-25% of TSC patients have no mutation on TSC1 and TSC2 geens and normal concevtional genetic testing result does not exclude TSC, or have any effect on the use of clinical diagnostic criteria to diagnose TSC. Clinical diagnostic criteria has major and minor features. Major features are (1) hypopigmented macules ≥ 3, at least 5 mm diameter, (2) angiofibromas (≥ 3) or fibrous cephalic plaque, (3) ungual fibromas (≥ 2), (4) shagreen patch, (5) multiple retinal hamartomas, (6) corticalal dysplasias (includes tubers and cerebral white matter radial migration lines), (7) subependymal nodules, (8) subependymal giant cell astrocytoma, (9) cardiac rabdomyoma, (10) lymphangioleiomyomatosis, and (11) angiomyolipomas (≥ 2). Minor features are (1) confetti skin lesions, (2) dental enamel pits, (3) intraoral fibroms, (4) retinal achromic patch, (5) multiple renal cysts, and (6) nonrenal hamartomas. Definite TSC indicates either 2 major features or 1 major feature and ≥ 2 minor features. Possible TSC indicates either one major feature or ≥ 2 minor features [4]. Our patient had 3 major features for the diagnosis of TSC which were retinal hamartoma, cardiac rhabdomyomas and cortical tubers.
Most of the studies suggest that in prenatal period, the initial typical findings of TSC is cardiac rhabdomyomas, most of which are asymptomatic. Cardiac rhabdomyomas are seen in approximately 50-70% of infants with TSC which may regress spontaneously [2]. In the early infancy, most of the patients present with a new onset seizure or infantile spasm whereas cardiac rhabdomyomas and hypopigmented macules also led to diagnosis. Polycystic kidney disease, gingival fibromas and retinal hamartomas are known as unusual presentations of TSC in early infancy [4]. Here, we report a newborn having cardiac rhabdomyomas and retinal hamartoma who underwent diagnostic evaluation because of family history of TSC.
Cardiac rhabdomyomas are the most common cardiac tumors in children with no malignant potential which are also known as benign foetal hamartomas. They are strongly associated with TSC, especially when existing multiple. Cardiac rhabdomyomas may occur in any location however, most frequenly located in the ventricles close to septum, intracavitary and often they are multiple. Although cardiac rhabdomyomas may be diagnosed prenatally and show signs of TSC in utero, it is considered as an important marker of TSC as confirmed right after delivery [4,7]. Diagnosed fetal cardiac rhabdomyomas which are generally multiple and large may decrease in size or even vanish in time [8]. Everolimus is a mammalian target of rapamycin (mTOR) inhibitors, which have been previously used to augment anticancer treatment regimens, prevent solid organ transplant rejection and neovascularization of artificial cardiac stents. It is approved for the treatment of subependymal giant cell astrocytomas and renal angiomyolipomas which are related with TSC. Also it has been shown that everolimus treatment has beneficial for the treatment of several other clinical manifestations of TSC such as cardiac rhabdomyomas, facial angiofibromas, epilepsy, and lymphangioleiomyomatosis. Surgical therapy is indicated in cases of heart failure or life threatening arrhythmias [9]. In our case, we diagnosed multiple cardiac intracavitary ovoid-spherical sessile masses which were located in throughout both ventricles close to apex. Since it was not causing life threatening hemodynamic failure, no surgery was indicated. The cardiologic evaluation of our patient was done monthly. There was no regression in the size and number of the cardiac masses. They were still enormously large, but not causing hemodynamic failure. There was a huge mass in the right ventricule outflow tract, interestingly not causing obstruction in pulmonary blood flow. The heart looked like a sack of potatoes. In order to check the effectiveness on decreasing the size of the huge masses, everolimus treatment was planned. When he was 5 months old, insurance procedures were completed and everolimus treatment was started. The sizes of the intracardiac masses were reduced approximately 1/3 of the initial sizes after 2 months. Comparing the first 5 months duration to the treatment period of last 2 months, there was a dramatically reduction in the size of masses. It was reported that during everolimus treatment stomatitis, mucositis, nasopharynngitis, acne-like skin lesions and hypercholesterolemia can be seen [9]. During the treatment no significant side effects were observed in our patient. There are only limited experience about everolimus treatment under 1 year of age. In our case, everolimus treatment was significantly effective on reducing the size of the masses without any side effects.
Hypomelanotic macules may ocur at birth or during the neonatal period as the earliest cutaneous lessions in TSC. The macules may present in any number and shape varying from 1 to 20. Presence of 3 or more hypomelanotic macules is considered as the major criteria of TSC [4,10]. Our patient didn’t have any hypomelanotic macules at birth and no lessions appeared during the follow up by wood’s light detection.
Infantile spasm or a new onset seizure are the earliest findings of the systemic manifestation of TSC in early infancy. Subependimal calcified nodule is considered the most common extracutaneous manifestation. Those nodules are observed by 2 years of age and by puberty they are present in almost all patients [10]. In addition to nodules, cortical tubers subependymal giant cell astrocytoma and abnormal white matter are known as cerebral pathologies of TSC. In the long term follow up, the patients with TSC may progress intellectual disability, autism spectrum disorder, epilepsy, learning and behaviour problems. In our case, we obtained multiple lessions reminding of tubers according to cranial MRI. The EEG findings showed parieto-central epileptic activity when he was 3 months old. We are following up the patient for the long term cerebral and neuropsychiatric problems.
The eye involment of TSC can be divided into 2 groups; retinal and non-retinal. The retinal lesions are known as astrocytic hamartomas. Hamartoma is a congenital, unilateral and hypopigmented lesion of the retina which is seen in the temporal macula. In most studies, the prevalance of retinal hamartoma ranges between 1.2-6.7% but Mayo Clinic gives an overall prevalence of 49% [10-12]. Non-retinal findings are angiofibromas of the eyelids, coloboma of the iris, lens and choroid, poliosis of the iris, papilloedema, sector iris depigmentation and strabismus. Ophtalmologic examination of our patient revealed a yellow colored and lobulated structure on right optic nerve as asrocytic hamartoma. The edema in the anterior palpebral soft tissue and a 5 mm mass in right orbit (on the posterior wall in choroidal region) were observed according to orbita MRI. During the follow up, the ophtalmologic examination revealed the same findings as before.
In conclusion, we present this case to alert the importance of antenatal diagnosis of TSC by fetal cardiac rhabdomyomas especially who has family history and to discuss the clinical features of TSC.
References
- Motoki N, Inaba Y, Matsuzaki S, Akazawa Y, Nishimura T, et al. (2016) Successful treatment of arrhythmia-induced cardiomyopathy in an infant with tuberous sclerosis complex. BMC Pediatr 16: 16.
- Jóźwiak S, Kotulska K, Kasprzyk-Obara J, Domańska-Pakieła D, Tomyn-Drabik M, et al. (2006) Clinical and genotype studies of cardiac tumors in 154 patients with tuberous sclerosis complex. Pediatrics 118: 1146-1151.
- Franz DN (2004) Non-neurologic manifestations of tuberous sclerosis complex. J Child Neurol 19: 690-698.
- Northrup H, Krueger DA, Roberds S, Smith K, Sampson J (2013) Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49: 243-254.
- Sampson JR, Scahill SJ, Stephenson JB, Mann L, Connor JM, et al. (1989) Genetic aspects of tuberous sclerosis in the west of Scotland. J Med Genet 26: 28-31.
- Van Slegtenhorst M, Nellist M, Nagelkarken B, Cheadle J, Snell R, et al. (1998) Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet 7: 1053-1057.
- Hodzig A, Maragnes P, Dupont-Chauvet P, Labombarda F (2013) Three dimensional echocardiographic assessment of multiple rhabdomyoma in newborn. Images Paediatr Cardiol 15: 1-4.
- Bader RS, Chitayat D, Kelly E, Ryan G, Smallhorn JF, et al. (2003) Fetal rhabdomyoma: parental diagnosis, clinical outcome and incidence of associated tuberous sclerosis complex. J Pediatr 143: 620-624.
- Capal JK, Franz DN (2016) Profile of everolimus in the treatment of tuberous sclerosis complex: an evidence-based review of its place in therapy. Neuropsychiatr Dis Treat 12: 2165-2172.
- Nath J, Dubey A, Pavan R (2015) Analysis of twenty pediatric cases of tuberous sclerosis complex: Are we doing enough?. Indian J Dermatol Venereol Leprol 81: 23-28.
- Rama Rao GR, Krishna Rao PV, Gopal K, Kumar YH, Ramachandra BV, et al. (2008) Forehead plaque: A cutaneous marker of CNS involvement in tuberous sclerosis. Indian J Dermatol Venereol Leprol 74: 28-31.
- Rowley SA, O’Callaghan FJ, Osborne JP (2002) Ophthalmic manifestations of tuberous sclerosis: a population based study. Br J Ophthalmol 85: 420-423.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences